- Apply liberally 15 minutes before sun exposure
- Use a water-resistant sunscreen if swimming or sweating: immediatelly after towel drying; At least every two hours.
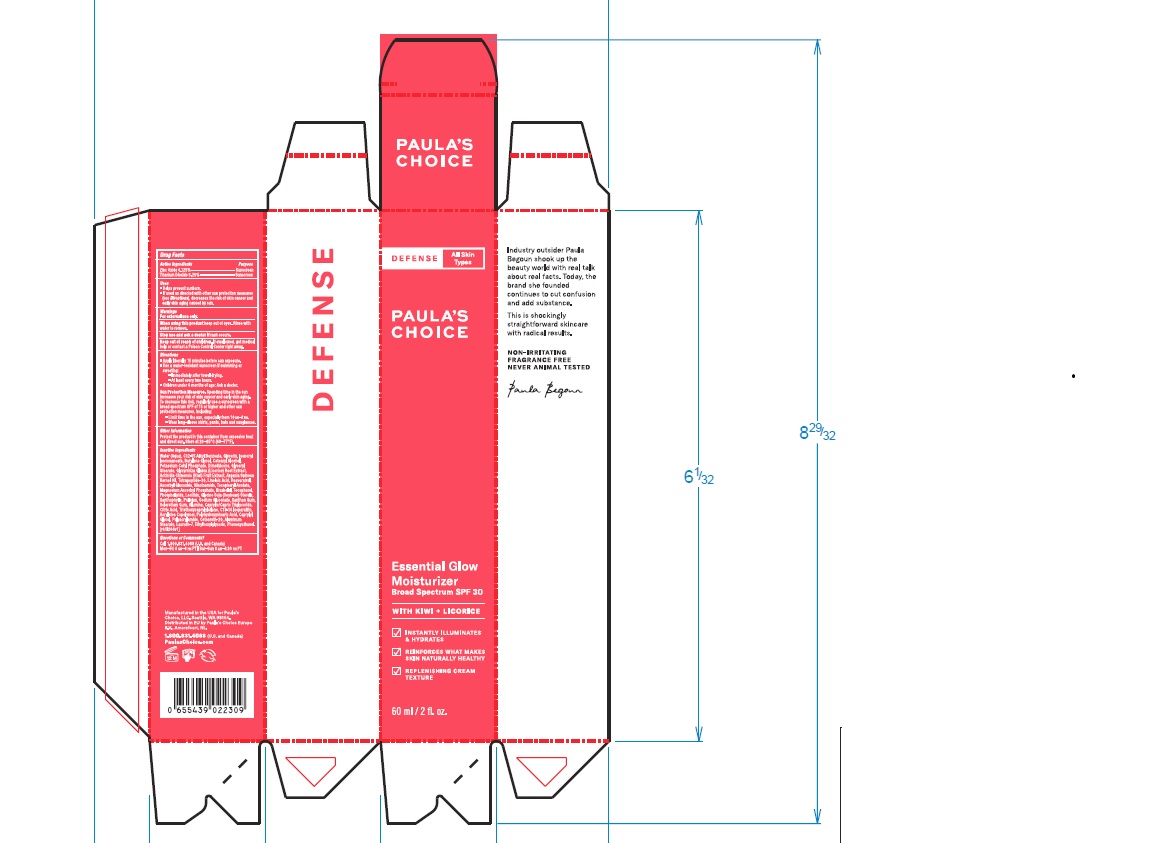
- Children under 6 months of age: Ask a doctor.
Water (Aqua), C12-15 Alkyl Benzoate, Glycerin, Isononyl Isononanoate, Butylene Glycol, Cetearyl Alcohol, Potassium Cetyl Phosphate, Dimethicone, Glyceryl Stearate, Glycyrrhiza Glabra (Licorice) Root Extract, Actinidia Chinensis (Kiwi) Fruit Extract, Argania Spinosa Kernel Oil, Tetrapeptide-30, Linoleic Acid, Resveratrol, Ascorbyl Glucoside, Niacinamide, Tocopheryl Acetate, Magnesium Ascorbyl Phosphate, Bisabolol, Tocopherol, Phospholipids, Lecithin, Glycine Soja (Soybean) Sterols, Xanthophylls, Pullulan, Sodium Gluconate, Xanthan Gum, Sclerotium Gum, Alumina, Caprylic/Capric Triglyceride, Citric Acid, Triethoxycaprylylsilane, C13-14 Isoparaffin, Acrylates Copolymer, Polyhydroxystearic Acid, Caprylyl Glycol, Polyacrylamide, Ceteareth-20, Aluminum Stearate, Laureth-7, Ethylhexylglycerin, Phenoxyethanol.