Label: ASPIRIN 81 MG- aspirin enteric coated tablets 81 mg tablet, delayed release
- NDC Code(s): 71406-128-10, 71406-128-12
- Packager: AACE Pharmaceuticals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
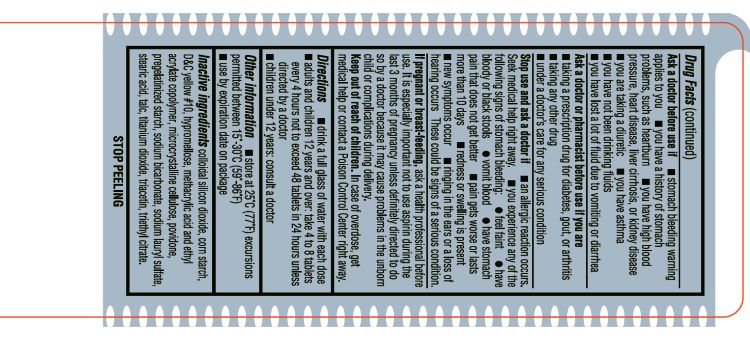
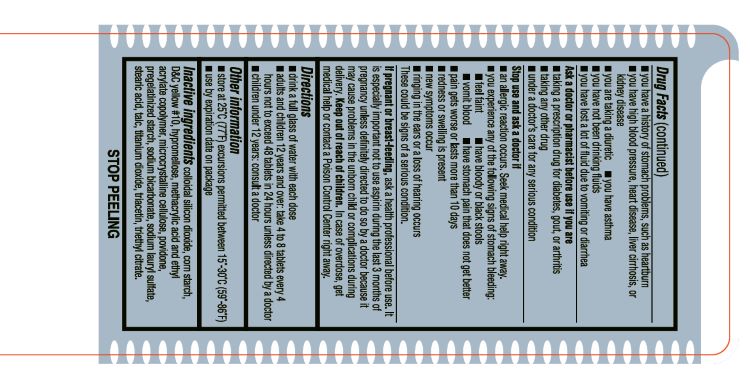
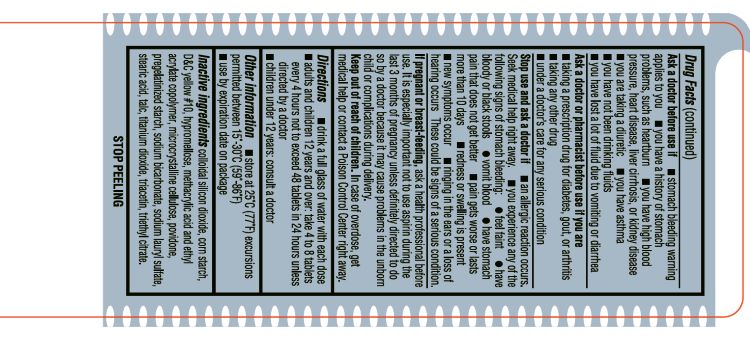
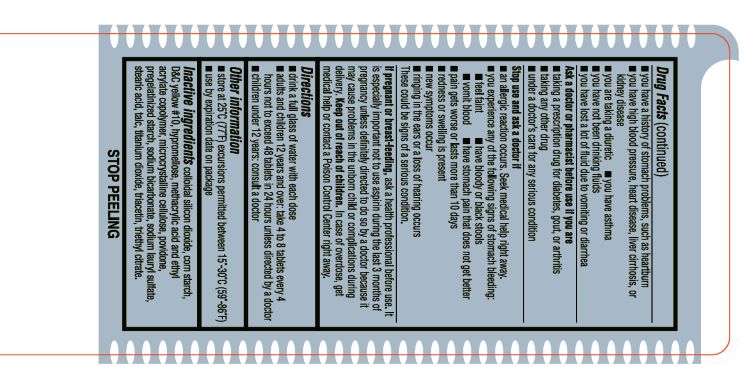
- INACTIVE INGREDIENT
- ACTIVE INGREDIENT
- ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- Aspirin Drug Facts
- KEEP OUT OF REACH OF CHILDREN
- PREGNANCY OR BREAST FEEDING
- PURPOSE
- STOP USE
- DOSAGE & ADMINISTRATION
- WARNINGS
- SPL UNCLASSIFIED SECTION
- OTHER SAFETY INFORMATION
- INDICATIONS & USAGE
-
PRINCIPAL DISPLAY PANEL
NDC 71406-128-12 AACE Pharmaceuticals - Adult Low Strength - Pain Reliever - Aspirin USP 81 mg (NSAID) Enteric Coated - 120 tablets - Compare to Active Ingredient in Aspirin Regimen BAYER®

 NDC 71406-128-10 AACE Pharmaceuticals - Adult Low Strength - Pain Reliever - Aspirin USP 81 mg (NSAID) Enteric Coated - 1000 tablets - Compare to Active Ingredient in Aspirin Regimen BAYER®
NDC 71406-128-10 AACE Pharmaceuticals - Adult Low Strength - Pain Reliever - Aspirin USP 81 mg (NSAID) Enteric Coated - 1000 tablets - Compare to Active Ingredient in Aspirin Regimen BAYER®

-
INGREDIENTS AND APPEARANCE
ASPIRIN 81 MG
aspirin enteric coated tablets 81 mg tablet, delayed releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71406-128 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 81 mg Inactive Ingredients Ingredient Name Strength TRIACETIN (UNII: XHX3C3X673) STEARIC ACID (UNII: 4ELV7Z65AP) POVIDONE (UNII: FZ989GH94E) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) SODIUM LAURYL SULFATE (UNII: 368GB5141J) MICROCRYSTALLINE CELLULOSE 102 (UNII: PNR0YF693Y) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:2) (UNII: XRK36F13ZZ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SODIUM BICARBONATE (UNII: 8MDF5V39QO) TALC (UNII: 7SEV7J4R1U) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) Product Characteristics Color yellow Score no score Shape ROUND Size 8mm Flavor Imprint Code S17 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71406-128-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 05/14/2021 2 NDC:71406-128-12 120 in 1 BOTTLE; Type 0: Not a Combination Product 05/14/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 05/03/2021 Labeler - AACE Pharmaceuticals, Inc. (080630748)