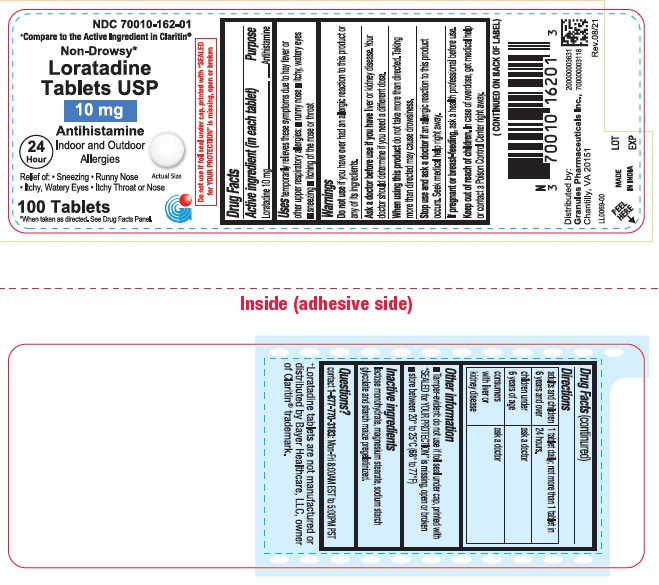
Label: LORATADINE tablet
- NDC Code(s): 70010-162-01, 70010-162-34
- Packager: Granules Pharmaceuticals Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT(S)
- PURPOSE
- USE(S)
- WARNINGS
- ASK A DOCTOR BEFORE USE IF
- WHEN USING THIS PRODUCT
- STOP USE AND ASK DOCTOR IF
- PREGNANCY/BREASTFEEDING
- KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS
- OTHER INFORMATION
- STORAGE
- INACTIVE INGREDIENTS
- QUESTIONS OR COMMENTS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LORATADINE
loratadine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70010-162 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) MAGNESIUM STEARATE (UNII: 70097M6I30) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color white (White to off white) Score no score Shape ROUND Size 6mm Flavor Imprint Code G;10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70010-162-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 11/17/2021 2 NDC:70010-162-34 300 in 1 BOTTLE; Type 0: Not a Combination Product 05/01/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210722 01/01/2020 Labeler - Granules Pharmaceuticals Inc. (079825711)