Label: ZILBRYSQ- zilucoplan injection, solution
- NDC Code(s): 50474-990-80, 50474-991-80, 50474-992-80
- Packager: UCB, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 13, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZILBRYSQ safely and effectively. See full prescribing information for ZILBRYSQ. ZILBRYSQ (zilucoplan) injection, for ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: SERIOUS MENINGOCOCCAL INFECTIONS
ZILBRYSQ, a complement inhibitor, increases the risk of serious infections caused by Neisseria meningitidis [see Warnings and Precautions (5.1)] . Life-threatening and fatal meningococcal infections have occurred in patients treated with complement inhibitors. These infections may become rapidly life-threatening or fatal if not recognized and treated early.
- Complete or update vaccination for meningococcal bacteria (for serogroups A, C, W, Y, and B) at least 2 weeks prior to the first dose of ZILBRYSQ, unless the risks of delaying therapy outweigh the risk of developing a serious infection. Comply with the most current Advisory Committee on Immunization Practices (ACIP) recommendations for vaccination against meningococcal bacteria in patients receiving a complement inhibitor. See Warnings and Precautions (5.1)for additional guidance on the management of the risk of serious infections caused by meningococcal bacteria.
- Patients receiving ZILBRYSQ are at increased risk for invasive disease caused by Neisseria meningitidis, even if they develop antibodies following vaccination. Monitor patients for early signs and symptoms of serious meningococcal infections and evaluate immediately if infection is suspected.
Because of the risk of serious meningococcal infections, ZILBRYSQ is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called ZILBRYSQ REMS [see Warnings and Precautions (5.2)] .
Close -
1 INDICATIONS AND USAGEZILBRYSQ is indicated for the treatment of generalized myasthenia gravis (gMG) in adult patients who are anti-acetylcholine receptor (AChR) antibody positive.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Vaccination and Prophylaxis for Meningococcal Infection - Vaccinate patients against meningococcal infection (serogroups A, C, W, Y, and B) according to current ACIP ...
-
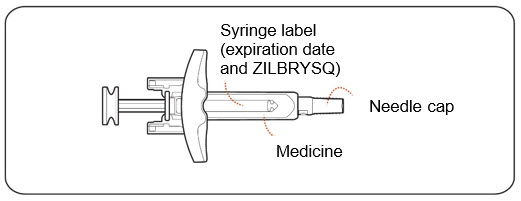
3 DOSAGE FORMS AND STRENGTHSInjection: 16.6 mg/0.416 mL, 23 mg/0.574 mL, or 32.4 mg/0.81 mL of zilucoplan as a clear to slightly opalescent, colorless solution in single-dose prefilled syringes.
-
4 CONTRAINDICATIONSZILBRYSQ is contraindicated for initiation in patients with unresolved serious - Neisseria meningitidisinfection - [see - Warnings and Precautions (5.1)] .
-
5 WARNINGS AND PRECAUTIONS5.1 Serious Meningococcal Infections - ZILBRYSQ, a complement inhibitor, increases a patient's susceptibility to serious, life-threatening, or fatal infections caused by meningococcal bacteria ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling: Serious Meningococcal Infections - [see - Warnings and ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on ZILBRYSQ use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or other adverse ...
-
11 DESCRIPTIONZilucoplan, a complement inhibitor, is a 15 amino-acid, synthetic macrocyclic peptide. The molecular formula of zilucoplan is C - 172H - 278N - 24O - 55in free acid form and its molecular ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Zilucoplan binds to the complement protein C5 and inhibits its cleavage to C5a and C5b, preventing the generation of the terminal complement complex, C5b-9. The precise ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Studies to assess the carcinogenic potential of zilucoplan have not been conducted. Mutagenesis - Zilucoplan ...
-
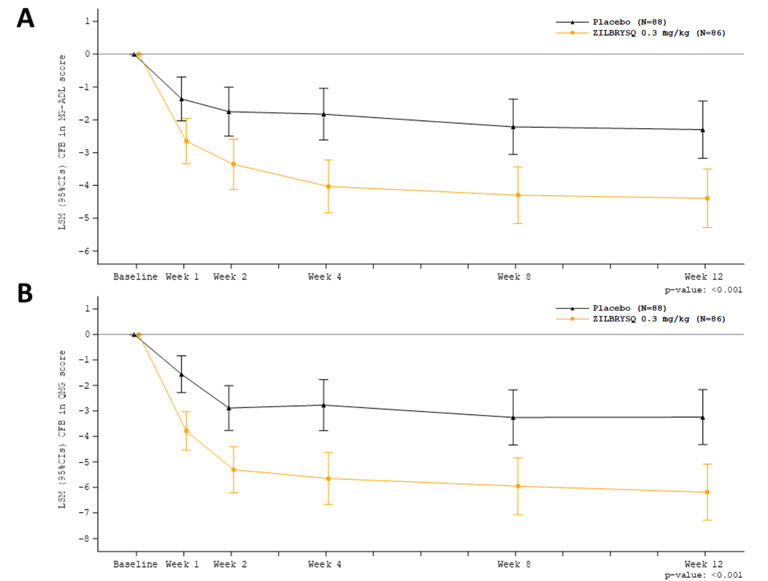
14 CLINICAL STUDIESThe efficacy of ZILBRYSQ for the treatment of generalized myasthenia gravis (gMG) in adult patients who are anti-AChR antibody positive was established in a 12-week, multicenter, randomized ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - ZILBRYSQ (zilucoplan) injection prefilled syringe contains a sterile, preservative-free, clear to slightly opalescent, colorless solution. Each single-dose prefilled syringe ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patients and/or caregivers to read FDA-approved patient labeling (Medication Guide and Instructions for Use) . Serious Meningococcal Infection - Advise patients of the risk of ...
-
SPL UNCLASSIFIED SECTIONManufactured for: UCB, Inc. 1950 Lake Park Drive - Smyrna, GA 30080 - ZILBRYSQ - ®is a registered trademark of the UCB Group of Companies. ©2024 UCB, Inc., Smyrna, GA 30080 ...
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration.Issued: 2/2025 MEDICATION GUIDE - ZILBRYSQ - ®(ZIL-brisk) (zilucoplan ...
-
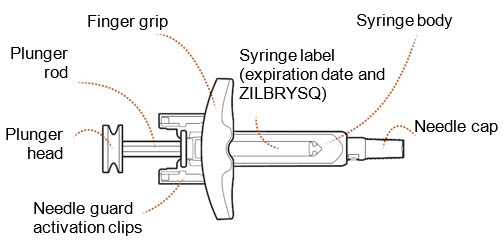
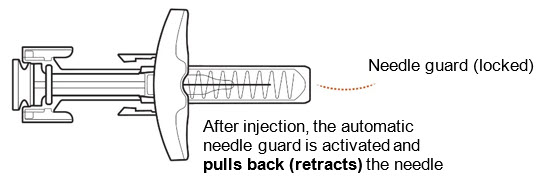
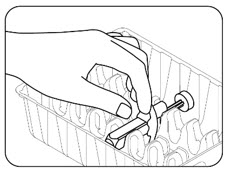
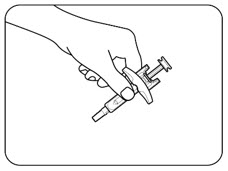
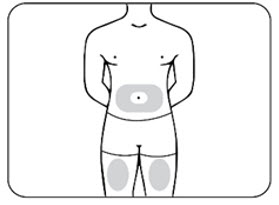
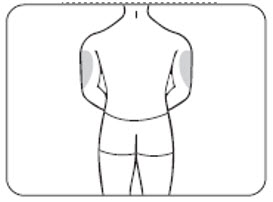
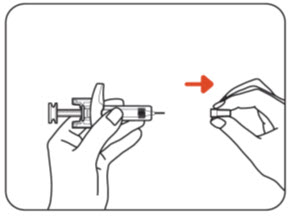
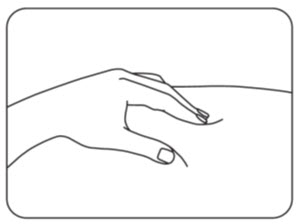
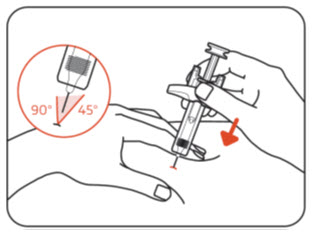
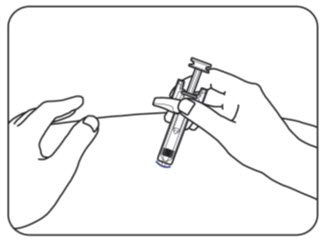
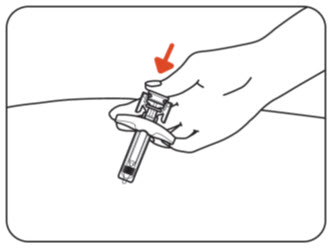
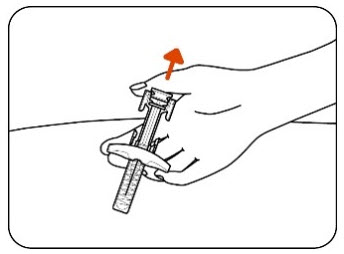
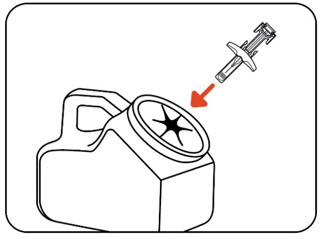
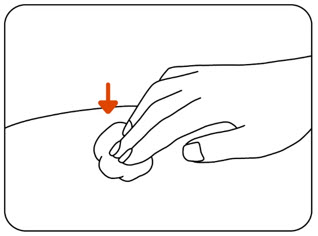
INSTRUCTIONS FOR USEZILBRYSQ - ®(ZIL-brisk) (zilucoplan) injection, for subcutaneous use - Single-Dose Prefilled Syringe - This Instructions for Use contains information on how to inject ...
-
PRINCIPAL DISPLAY PANEL - 16.6 mg/0.416 mL Syringe Carton BoxNDC 50474-990-80 - Rx only - Do not accept - if seal is missing or broken. Lift here to open. ⇾ ZILBRYSQ - ® (zilucoplan) Injection - 16.6 mg/0.416 mL - For Subcutaneous Use ...
-
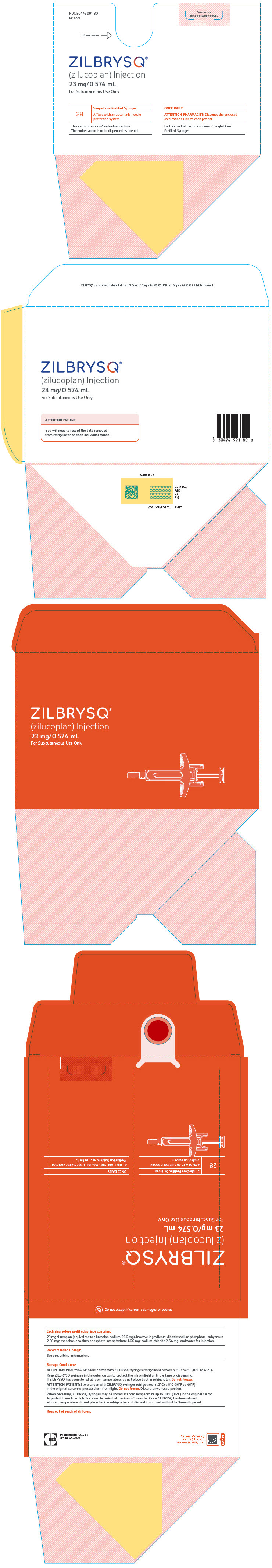
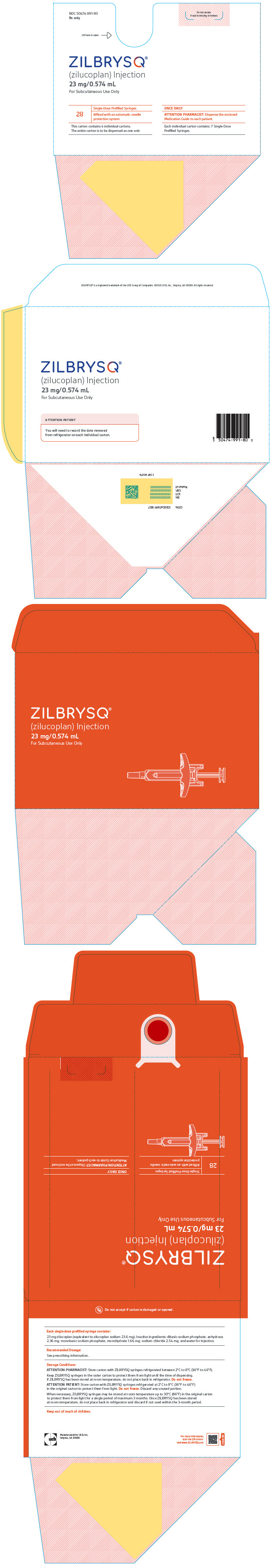
PRINCIPAL DISPLAY PANEL - 23 mg/0.574 mL Syringe Carton BoxNDC 50474-991-80 - Rx only - Do not accept - if seal is missing or broken. Lift here to open. ⇾ ZILBRYSQ - ® (zilucoplan) Injection - 23 mg/0.574 mL - For Subcutaneous Use ...
-
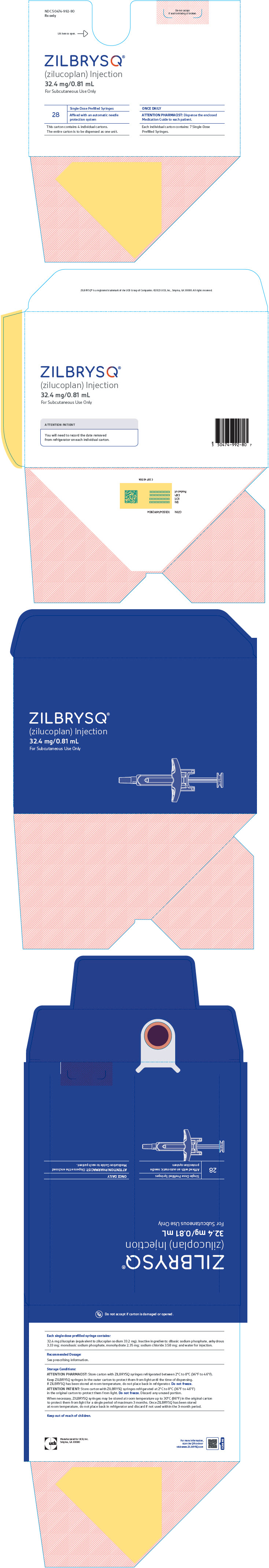
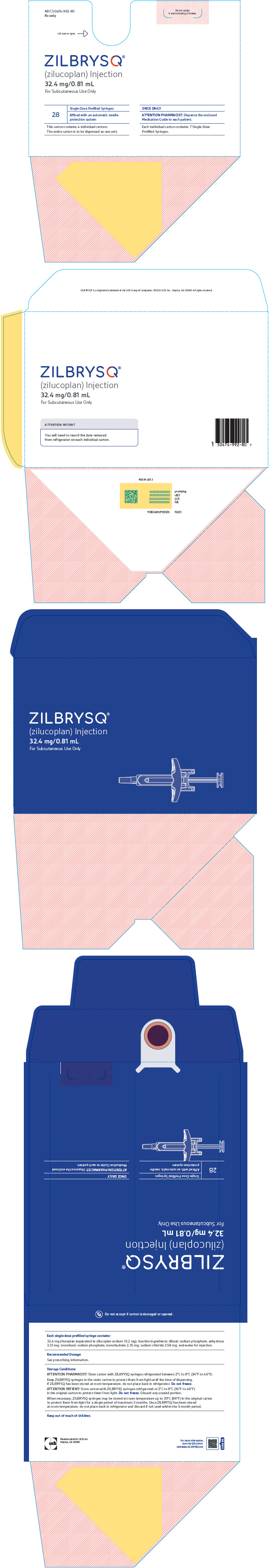
PRINCIPAL DISPLAY PANEL - 32.4 mg/0.81 mL Syringe Carton BoxNDC 50474-992-80 - Rx only - Do not accept - if seal is missing or broken. Lift here to open. ⇾ ZILBRYSQ - ® (zilucoplan) Injection - 32.4 mg/0.81 mL - For Subcutaneous Use ...
-
INGREDIENTS AND APPEARANCEProduct Information