Label: ENHERTU- fam-trastuzumab deruxtecan-nxki injection, powder, lyophilized, for solution
- NDC Code(s): 65597-406-01
- Packager: Daiichi Sankyo Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated January 28, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ENHERTU safely and effectively. See full prescribing information for ENHERTU. ENHERTU® (fam-trastuzumab deruxtecan-nxki) for ...These highlights do not include all the information needed to use ENHERTU safely and effectively. See full prescribing information for ENHERTU.
ENHERTU® (fam-trastuzumab deruxtecan-nxki) for injection, for intravenous use
Initial U.S. Approval: 2019WARNING: INTERSTITIAL LUNG DISEASE and EMBRYO-FETAL TOXICITY
See full prescribing information for complete boxed warning.
- Interstitial lung disease (ILD) and pneumonitis, including fatal cases, have been reported with ENHERTU. Monitor for and promptly investigate signs and symptoms including cough, dyspnea, fever, and other new or worsening respiratory symptoms. Permanently discontinue ENHERTU in all patients with Grade 2 or higher ILD/pneumonitis. Advise patients of the risk and to immediately report symptoms. (2.3, 5.1)
- Exposure to ENHERTU during pregnancy can cause embryo-fetal harm. Advise patients of these risks and the need for effective contraception. (5.4, 8.1, 8.3)
RECENT MAJOR CHANGES
Indications and Usage (1.2) 01/2025 Indications and Usage (1.1, 1.4, 1.5) 04/2024 Dosage and Administration (2.1, 2.2) 01/2025 Dosage and Administration (2.1, 2.2, 2.3, 2.4) 04/2024 Dosage and Administration (2.2, 2.4) 02/2024 Warnings and Precautions (5.1, 5.2, 5.3) 01/2025 Warnings and Precautions (5.1, 5.2, 5.3) 04/2024 Warnings and Precautions (5.1, 5.2, 5.3) 02/2024 INDICATIONS AND USAGE
ENHERTU is a HER2-directed antibody and topoisomerase inhibitor conjugate indicated for the treatment of:
- adult patients with unresectable or metastatic HER2-positive (IHC 3+ or ISH positive) breast cancer who have received a prior anti-HER2-based regimen either:
- in the metastatic setting, or
- in the neoadjuvant or adjuvant setting and have developed disease recurrence during or within six months of completing therapy. (1.1)
- adult patients with unresectable or metastatic
- Hormone receptor (HR)-positive, HER2-low (IHC 1+ or IHC 2+/ISH-) or HER2-ultralow (IHC 0 with membrane staining) breast cancer, as determined by an FDA-approved test, that has progressed on one or more endocrine therapies in the metastatic setting. (1.2)
- HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer, as determined by an FDA-approved test, who have received a prior chemotherapy in the metastatic setting; or developed disease recurrence during or within 6 months of completing adjuvant chemotherapy. (1.2)
- adult patients with unresectable or metastatic non-small cell lung cancer (NSCLC) whose tumors have activating HER2 (ERBB2) mutations, as detected by an FDA-approved test, and who have received a prior systemic therapy.* (1.3)
- adult patients with locally advanced or metastatic HER2-positive (IHC 3+ or IHC 2+/ISH positive) gastric or gastroesophageal junction adenocarcinoma who have received a prior trastuzumab-based regimen. (1.4)
- adult patients with unresectable or metastatic HER2-positive (IHC 3+) solid tumors who have received prior systemic treatment and have no satisfactory alternative treatment options.* (1.5)
* These indications are approved under accelerated approval based on objective response rate and duration of response. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial. (14.3, 14.5)
DOSAGE AND ADMINISTRATION
- Do not substitute ENHERTU for or with trastuzumab or ado-trastuzumab emtansine. (2.2, 2.4)
- For intravenous infusion only. Do not administer as an intravenous push or bolus. DO NOT use Sodium Chloride Injection, USP. (2.4)
- Premedicate for prevention of chemotherapy-induced nausea and vomiting. (2.2)
- The recommended dosage of ENHERTU for HER2-positive, HER2-low, or HER2-ultralow breast cancer, HER2-mutant NSCLC, and HER2-positive (IHC 3+) solid tumors is 5.4 mg/kg given as an intravenous infusion once every 3 weeks (21-day cycle) until disease progression or unacceptable toxicity. (2.2, 2.3)
- The recommended dosage of ENHERTU for HER2-positive gastric cancer is 6.4 mg/kg given as an intravenous infusion once every 3 weeks (21-day cycle) until disease progression or unacceptable toxicity. (2.2, 2.3)
- Management of adverse reactions (ILD, neutropenia, thrombocytopenia, or left ventricular dysfunction) may require temporary interruption, dose reduction, or discontinuation of ENHERTU. (2.3)
DOSAGE FORMS AND STRENGTHS
For injection: 100 mg lyophilized powder in a single-dose vial (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Neutropenia: Monitor complete blood counts prior to initiation of ENHERTU and prior to each dose, and as clinically indicated. Manage through treatment interruption or dose reduction. (2.3, 5.2)
- Left Ventricular Dysfunction: Assess LVEF prior to initiation of ENHERTU and at regular intervals during treatment as clinically indicated. Manage through treatment interruption or discontinuation. Permanently discontinue ENHERTU in patients with symptomatic congestive heart failure (CHF). (2.3, 5.3)
ADVERSE REACTIONS
The most common adverse reactions (≥20%), including laboratory abnormalities, in patients with:
- HER2-positive, HER2-low, and HER2-ultralow metastatic breast cancer, HER2-mutant NSCLC, and HER2-positive (including IHC 3+) solid tumors are decreased white blood cell count, nausea, decreased hemoglobin, decreased neutrophil count, decreased lymphocyte count, fatigue, decreased platelet count, increased aspartate aminotransferase, increased alanine aminotransferase, increased blood alkaline phosphatase, vomiting, alopecia, constipation, decreased blood potassium, decreased appetite, diarrhea, and musculoskeletal pain. (6.1)
- HER2-positive gastric cancer are decreased hemoglobin, decreased white blood cell count, decreased neutrophil count, decreased lymphocyte count, decreased platelet count, nausea, decreased appetite, increased aspartate aminotransferase, fatigue, increased blood alkaline phosphatase, increased alanine aminotransferase, diarrhea, decreased blood potassium, vomiting, constipation, increased blood bilirubin, pyrexia, and alopecia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Daiichi Sankyo, Inc. at 1-877-437-7763 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 1/2025
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: INTERSTITIAL LUNG DISEASE and EMBRYO-FETAL TOXICITY
1 INDICATIONS AND USAGE
1.1 HER2-Positive Metastatic Breast Cancer
1.2 HER2-Low and HER2-Ultralow Metastatic Breast Cancer
1.3 HER2-Mutant Unresectable or Metastatic Non-Small Cell Lung Cancer
1.4 HER2-Positive Locally Advanced or Metastatic Gastric Cancer
1.5 HER2-Positive (IHC 3+) Unresectable or Metastatic Solid Tumors
2 DOSAGE AND ADMINISTRATION
2.1 Patient Selection
2.2 Recommended Dosage and Schedules
2.3 Dosage Modifications
2.4 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Interstitial Lung Disease/Pneumonitis
5.2 Neutropenia
5.3 Left Ventricular Dysfunction
5.4 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 HER2-Positive Metastatic Breast Cancer
14.2 HER2-Low and HER2-Ultralow Metastatic Breast Cancer
14.3 HER2-Mutant Unresectable or Metastatic Non-Small Cell Lung Cancer
14.4 HER2-Positive Locally Advanced or Metastatic Gastric Cancer
14.5 HER2-Positive (IHC 3+) Unresectable or Metastatic Solid Tumors
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: INTERSTITIAL LUNG DISEASE and EMBRYO-FETAL TOXICITY
- Interstitial Lung Disease (ILD) and pneumonitis, including fatal cases, have been reported with ENHERTU. Monitor for and promptly investigate signs and symptoms including cough, dyspnea, fever, and other new or worsening respiratory symptoms. Permanently discontinue ENHERTU in all patients with Grade 2 or higher ILD/pneumonitis. Advise patients of the risk and the need to immediately report symptoms [see Dosage and Administration (2.3), Warnings and Precautions (5.1)].
- Embryo-Fetal Toxicity: Exposure to ENHERTU during pregnancy can cause embryo-fetal harm. Advise patients of these risks and the need for effective contraception [see Warnings and Precautions (5.4), Use in Specific Populations (8.1, 8.3)].
-
1 INDICATIONS AND USAGE1.1 HER2-Positive Metastatic Breast Cancer - ENHERTU is indicated for the treatment of adult patients with unresectable or metastatic HER2-positive (IHC 3+ or ISH positive) breast cancer who ...
1.1 HER2-Positive Metastatic Breast Cancer
ENHERTU is indicated for the treatment of adult patients with unresectable or metastatic HER2-positive (IHC 3+ or ISH positive) breast cancer who have received a prior anti-HER2-based regimen either:
- in the metastatic setting, or
- in the neoadjuvant or adjuvant setting and have developed disease recurrence during or within six months of completing therapy.
1.2 HER2-Low and HER2-Ultralow Metastatic Breast Cancer
ENHERTU is indicated for the treatment of adult patients with unresectable or metastatic
- Hormone receptor (HR)-positive HER2-low (IHC 1+ or IHC 2+/ISH-) or HER2-ultralow (IHC 0 with membrane staining) breast cancer, as determined by an FDA-approved test, that has progressed on one or more endocrine therapies in the metastatic setting [see Dosage and Administration (2.1)].
- HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer, as determined by an FDA-approved test, who have received a prior chemotherapy in the metastatic setting or developed disease recurrence during or within 6 months of completing adjuvant chemotherapy [see Dosage and Administration (2.1)].
1.3 HER2-Mutant Unresectable or Metastatic Non-Small Cell Lung Cancer
ENHERTU is indicated for the treatment of adult patients with unresectable or metastatic non-small cell lung cancer (NSCLC) whose tumors have activating HER2 (ERBB2) mutations, as detected by an FDA-approved test, and who have received a prior systemic therapy.
This indication is approved under accelerated approval based on objective response rate and duration of response [see Clinical Studies (14.3)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
1.4 HER2-Positive Locally Advanced or Metastatic Gastric Cancer
ENHERTU is indicated for the treatment of adult patients with locally advanced or metastatic HER2-positive (IHC 3+ or IHC 2+/ISH positive) gastric or gastroesophageal junction (GEJ) adenocarcinoma who have received a prior trastuzumab-based regimen.
Close1.5 HER2-Positive (IHC 3+) Unresectable or Metastatic Solid Tumors
ENHERTU is indicated for the treatment of adult patients with unresectable or metastatic HER2-positive (IHC 3+) solid tumors who have received prior systemic treatment and have no satisfactory alternative treatment options.
This indication is approved under accelerated approval based on objective response rate and duration of response [see Clinical Studies (14.5)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
-
2 DOSAGE AND ADMINISTRATION2.1 Patient Selection - HER2-Low or HER2-Ultralow Unresectable or Metastatic Breast Cancer - Select patients for treatment of unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) or ...
2.1 Patient Selection
HER2-Low or HER2-Ultralow Unresectable or Metastatic Breast Cancer
Select patients for treatment of unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) or HER2-ultralow (IHC 0 with membrane staining) breast cancer with ENHERTU based on HER2 expression [see Clinical Studies (14.2)].
HER2-Mutant Unresectable or Metastatic NSCLC
Select patients for the treatment of unresectable or metastatic HER2-mutant NSCLC with ENHERTU based on the presence of activating HER2 (ERBB2) mutations in tumor or plasma specimens [see Clinical Studies (14.3)]. If no mutation is detected in a plasma specimen, test tumor tissue.
HER2-Positive Locally Advanced or Metastatic Gastric Cancer
Select patients with locally advanced or metastatic HER2-positive gastric cancer based on HER2 protein overexpression or HER2 gene amplification (IHC 3+ or IHC 2+/ISH positive). Reassess HER2 status if it is feasible to obtain a new tumor specimen after prior trastuzumab-based therapy and before treatment with ENHERTU.
HER2-Positive (IHC 3+) Unresectable or Metastatic Solid Tumors
Select patients for treatment of unresectable or metastatic solid tumors with ENHERTU based on HER2-positive (IHC 3+) specimens [see Clinical Studies (14.5)]. An FDA-approved test for the detection of HER2-positive (IHC 3+) solid tumors for treatment with ENHERTU is not currently available.
2.2 Recommended Dosage and Schedules
Do not substitute ENHERTU for or with trastuzumab or ado-trastuzumab emtansine.
Slow or interrupt the infusion rate if the patient develops infusion-related symptoms.
Permanently discontinue ENHERTU in case of severe infusion reactions.
Premedication
ENHERTU is highly emetogenic [see Adverse Reactions (6.1)], which includes delayed nausea and/or vomiting. Administer prophylactic antiemetic medications per local institutional guidelines for prevention of chemotherapy-induced nausea and vomiting.
Recommended Dosage for HER2-Positive, HER2-Low, or HER2-Ultralow Metastatic Breast Cancer, HER2-Mutant Unresectable or Metastatic NSCLC, and HER2-Positive (IHC 3+) Unresectable or Metastatic Solid Tumors.
The recommended dosage of ENHERTU is 5.4 mg/kg given as an intravenous infusion once every 3 weeks (21-day cycle) until disease progression or unacceptable toxicity.
2.3 Dosage Modifications
Management of adverse reactions may require temporary interruption, dose reduction, or treatment discontinuation of ENHERTU as described in Tables 1 and 2.
Do not re-escalate the ENHERTU dose after a dose reduction is made.
If a planned dose is delayed or missed, administer as soon as possible; do not wait until the next planned cycle. Adjust the schedule of administration to maintain a 3-week interval between doses. Administer the infusion at the dose and rate the patient tolerated in the most recent infusion.
Table 1: Dosage Reduction Schedule Dose Reduction Schedule Breast Cancer, NSCLC, and IHC 3+ Solid Tumors Gastric Cancer Recommended starting dose 5.4 mg/kg 6.4 mg/kg First dose reduction 4.4 mg/kg 5.4 mg/kg Second dose reduction 3.2 mg/kg 4.4 mg/kg Requirement for further dose reduction Discontinue treatment. Discontinue treatment. Table 2: Dosage Modifications for Adverse Reactions Adverse Reaction Severity Treatment Modification Toxicity grades are in accordance with National Cancer Institute Common Terminology Criteria for Adverse Events Version 5.0 (NCI CTCAE v.5.0). Interstitial Lung Disease (ILD)/ Pneumonitis
[see Warnings and Precautions (5.1)]Asymptomatic ILD/pneumonitis
(Grade 1)Interrupt ENHERTU until resolved to Grade 0, then: - if resolved in 28 days or less from date of onset, maintain dose.
- if resolved in greater than 28 days from date of onset, reduce dose one level (see Table 1).
- consider corticosteroid treatment as soon as ILD/pneumonitis is suspected.
Symptomatic ILD/pneumonitis
(Grade 2 or greater)- Permanently discontinue ENHERTU.
- Promptly initiate corticosteroid treatment as soon as ILD/pneumonitis is suspected.
Neutropenia
[see Warnings and Precautions (5.2)]Grade 3 (less than 1.0 to 0.5 × 109/L) - Interrupt ENHERTU until resolved to Grade 2 or less, then maintain dose.
Grade 4 (less than 0.5 × 109/L) - Interrupt ENHERTU until resolved to Grade 2 or less.
- Reduce dose by one level (see Table 1).
Febrile Neutropenia
[see Warnings and Precautions (5.2)]Absolute neutrophil count of less than 1.0 × 109/L and temperature greater than 38.3°C or a sustained temperature of 38°C or greater for more than one hour - Interrupt ENHERTU until resolved.
- Reduce dose by one level (see Table 1).
Thrombocytopenia
[see Adverse Reactions (6.1)]Grade 3 (platelets less than 50 to 25 × 109/L) - Interrupt ENHERTU until resolved to Grade 1 or less, then maintain dose.
Grade 4 (platelets less than 25 × 109/L) - Interrupt ENHERTU until resolved to Grade 1 or less.
- Reduce dose by one level (see Table 1).
Left Ventricular Dysfunction
[see Warnings and Precautions (5.3)]LVEF greater than 45% and absolute decrease from baseline is 10% to 20% - Continue treatment with ENHERTU.
LVEF 40% to 45% And absolute decrease from baseline is less than 10% - Continue treatment with ENHERTU.
- Repeat LVEF assessment within 3 weeks.
And absolute decrease from baseline is 10% to 20% - Interrupt ENHERTU.
- Repeat LVEF assessment within 3 weeks.
- If LVEF has not recovered to within 10% from baseline, permanently discontinue ENHERTU.
- If LVEF recovers to within 10% from baseline, resume treatment with ENHERTU at the same dose.
LVEF less than 40% or absolute decrease from baseline is greater than 20% - Interrupt ENHERTU.
- Repeat LVEF assessment within 3 weeks.
- If LVEF of less than 40% or absolute decrease from baseline of greater than 20% is confirmed, permanently discontinue ENHERTU.
Symptomatic congestive heart failure (CHF) - Permanently discontinue ENHERTU.
Close2.4 Preparation and Administration
In order to prevent medication errors, check the vial labels to ensure that the drug being prepared and administered is ENHERTU (fam-trastuzumab deruxtecan-nxki) and not trastuzumab or ado-trastuzumab emtansine.
Reconstitute and further dilute ENHERTU prior to intravenous infusion. Use appropriate aseptic technique.
ENHERTU (fam-trastuzumab deruxtecan-nxki) is a hazardous drug. Follow applicable special handling and disposal procedures.1
Reconstitution
- Reconstitute immediately before dilution.
- More than one vial may be needed for a full dose. Calculate the dose (mg), the total volume of reconstituted ENHERTU solution required, and the number of vial(s) of ENHERTU needed [see Dosage and Administration (2.2)].
- Reconstitute each 100 mg vial by using a sterile syringe to slowly inject 5 mL of Sterile Water for Injection, USP into each vial to obtain a final concentration of 20 mg/mL.
- Swirl the vial gently until completely dissolved. Do not shake.
- If not used immediately, store the reconstituted ENHERTU vials in a refrigerator at 2ºC to 8ºC (36ºF to 46ºF) for up to 24 hours from the time of reconstitution, protect the vial from light. Do not freeze.
- The product does not contain a preservative. Discard unused ENHERTU after 24 hours refrigerated.
Dilution
- Withdraw the calculated amount from the vial(s) using a sterile syringe. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. The solution should be clear and colorless to light yellow. Do not use if visible particles are observed or if the solution is cloudy or discolored.
- Dilute the calculated volume of reconstituted ENHERTU in an intravenous infusion bag containing 100 mL of 5% Dextrose Injection, USP. DO NOT use Sodium Chloride Injection, USP. ENHERTU is compatible with an infusion bag made of polyvinylchloride or polyolefin (copolymer of ethylene and polypropylene).
- Gently invert the infusion bag to thoroughly mix the solution. Do not shake.
- Cover the infusion bag to protect from light.
- Discard any unused portion left in the vials.
Administration
- If not used immediately, store the diluted ENHERTU in a refrigerator at 2ºC to 8ºC (36ºF to 46ºF) for up to 24 hours or at room temperature between 20ºC to 25ºC (68ºF to 77 ºF) for up to 4 hours including preparation and infusion time.
- Protect from light. Do not freeze.
- The maximum time from reconstitution of the vial through the end of administration should not exceed 24 hours.
- If the prepared infusion solution was stored refrigerated (2ºC to 8ºC [36ºF to 46ºF]), allow the solution to reach room temperature prior to administration. Cover the infusion bag to protect from light.
- Administer ENHERTU as an intravenous infusion only with an infusion set made of polyolefin or polybutadiene.
- Administer ENHERTU with a 0.20 or 0.22 micron in-line polyethersulfone (PES) or polysulfone (PS) filter.
- Do NOT administer as an intravenous push or bolus.
- Cover the infusion bag to protect from light during administration.
- Do not mix ENHERTU with other drugs or administer other drugs through the same intravenous line.
- First infusion: Administer infusion over 90 minutes.
- Subsequent infusions: Administer over 30 minutes if prior infusions were well tolerated.
-
3 DOSAGE FORMS AND STRENGTHSFor injection: 100 mg of fam-trastuzumab deruxtecan-nxki as a white to yellowish white lyophilized powder in a single-dose vial for reconstitution and further dilution
For injection: 100 mg of fam-trastuzumab deruxtecan-nxki as a white to yellowish white lyophilized powder in a single-dose vial for reconstitution and further dilution
Close -
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Interstitial Lung Disease/Pneumonitis - Severe, life-threatening, or fatal interstitial lung disease (ILD), including pneumonitis, can occur in patients treated with ENHERTU [see Adverse ...
5.1 Interstitial Lung Disease/Pneumonitis
Severe, life-threatening, or fatal interstitial lung disease (ILD), including pneumonitis, can occur in patients treated with ENHERTU [see Adverse Reactions (6.1)]. A higher incidence of Grade 1 and 2 ILD/pneumonitis has been observed in patients with moderate renal impairment.
Advise patients to immediately report cough, dyspnea, fever, and/or any new or worsening respiratory symptoms. Monitor patients for signs and symptoms of ILD. Promptly investigate evidence of ILD. Evaluate patients with suspected ILD by radiographic imaging. Consider consultation with a pulmonologist. For asymptomatic (Grade 1) ILD, consider corticosteroid treatment (e.g., ≥0.5 mg/kg/day prednisolone or equivalent). Withhold ENHERTU until recovery [see Dosage and Administration (2.3)]. In cases of symptomatic ILD (Grade 2 or greater), promptly initiate systemic corticosteroid treatment (e.g., ≥1 mg/kg/day prednisolone or equivalent) and continue for at least 14 days followed by gradual taper for at least 4 weeks. Permanently discontinue ENHERTU in patients who are diagnosed with symptomatic (Grade 2 or greater) ILD [see Dosage and Administration (2.3)].
HER2-Positive, HER2-Low, and HER2-Ultralow Metastatic Breast Cancer, HER2-Mutant NSCLC, and Solid Tumors (Including IHC 3+) (5.4 mg/kg)
In patients with metastatic breast cancer, HER2-mutant NSCLC, and other solid tumors treated with ENHERTU 5.4 mg/kg, ILD occurred in 12% of patients. Median time to first onset was 5.5 months (range: 0.9 to 31.5). Fatal outcomes due to ILD and/or pneumonitis occurred in 0.9% of patients treated with ENHERTU.
5.2 Neutropenia
Severe neutropenia, including febrile neutropenia, can occur in patients treated with ENHERTU.
Monitor complete blood counts prior to initiation of ENHERTU and prior to each dose, and as clinically indicated. Based on the severity of neutropenia, ENHERTU may require dose interruption or reduction [see Dosage and Administration (2.3)].
HER2-Positive, HER2-Low, and HER2-Ultralow Metastatic Breast Cancer, HER2-Mutant NSCLC, and Solid Tumors (Including IHC 3+) (5.4 mg/kg)
In patients with metastatic breast cancer, HER2-mutant NSCLC, and other solid tumors treated with ENHERTU 5.4 mg/kg, a decrease in neutrophil count was reported in 65% of patients. Nineteen percent had Grade 3 or 4 decreased neutrophil count. Median time to first onset of decreased neutrophil count was 22 days (range: 2 to 939). Febrile neutropenia was reported in 1.2% of patients.
HER2-Positive Locally Advanced or Metastatic Gastric Cancer (6.4 mg/kg)
In patients with locally advanced or metastatic HER2-positive gastric or GEJ adenocarcinoma treated with ENHERTU 6.4 mg/kg, a decrease in neutrophil count was reported in 72% of patients. Fifty-one percent had Grade 3 or 4 decreased neutrophil count. Median time to first onset of decreased neutrophil count was 16 days (range: 4 to 187). Febrile neutropenia was reported in 4.8% of patients.
5.3 Left Ventricular Dysfunction
Patients treated with ENHERTU may be at increased risk of developing left ventricular dysfunction. Left ventricular ejection fraction (LVEF) decrease has been observed with anti-HER2 therapies, including ENHERTU.
Assess LVEF prior to initiation of ENHERTU and at regular intervals during treatment as clinically indicated. Manage LVEF decrease through treatment interruption. Permanently discontinue ENHERTU if LVEF of less than 40% or absolute decrease from baseline of greater than 20% is confirmed. Permanently discontinue ENHERTU in patients with symptomatic congestive heart failure (CHF) [see Dosage and Administration (2.3)].
Treatment with ENHERTU has not been studied in patients with a history of clinically significant cardiac disease or LVEF less than 50% prior to initiation of treatment.
HER2-Positive, HER2-Low, and HER2-Ultralow Metastatic Breast Cancer, HER2-Mutant NSCLC, and Solid Tumors (Including IHC 3+) (5.4 mg/kg)
In patients with metastatic breast cancer, HER2-mutant NSCLC, and other solid tumors treated with ENHERTU 5.4 mg/kg, LVEF decrease was reported in 4.6% of patients, of which 0.6% were Grade 3 or 4.
HER2-Positive Locally Advanced or Metastatic Gastric Cancer (6.4 mg/kg)
In patients with locally advanced or metastatic HER2-positive gastric or GEJ adenocarcinoma treated with ENHERTU 6.4 mg/kg, no clinical adverse events of heart failure were reported; however, on echocardiography, 8% were found to have asymptomatic Grade 2 decrease in LVEF.
Close5.4 Embryo-Fetal Toxicity
Based on its mechanism of action, ENHERTU can cause fetal harm when administered to a pregnant woman. In postmarketing reports, use of a HER2-directed antibody during pregnancy resulted in cases of oligohydramnios manifesting as fatal pulmonary hypoplasia, skeletal abnormalities, and neonatal death. Based on its mechanism of action, the topoisomerase inhibitor component of ENHERTU, DXd, can also cause embryo-fetal harm when administered to a pregnant woman because it is genotoxic and targets actively dividing cells [see Use in Specific Populations (8.1), Clinical Pharmacology (12.1), Nonclinical Toxicology (13.1)]. Advise patients of the potential risks to a fetus.
Verify the pregnancy status of females of reproductive potential prior to the initiation of ENHERTU. Advise females of reproductive potential to use effective contraception during treatment and for 7 months after the last dose of ENHERTU. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with ENHERTU and for 4 months after the last dose of ENHERTU [see Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Interstitial Lung Disease/Pneumonitis [see Warnings and Precautions (5.1)] Neutropenia [see ...
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Interstitial Lung Disease/Pneumonitis [see Warnings and Precautions (5.1)]
- Neutropenia [see Warnings and Precautions (5.2)]
- Left Ventricular Dysfunction [see Warnings and Precautions (5.3)]
Close6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
HER2-Positive, HER2-Low, and HER2-Ultralow Metastatic Breast Cancer, HER2-Mutant NSCLC, and Solid Tumors (Including IHC 3+) (5.4 mg/kg)
The pooled safety population described in WARNINGS and PRECAUTIONS reflects exposure to ENHERTU 5.4 mg/kg intravenously every 3 weeks in 2233 patients in Study DS8201-A-J101 (NCT02564900), DESTINY-Breast01, DESTINY-Breast02, DESTINY-Breast03, DESTINY-Breast04, DESTINY-Breast06, DESTINY-Lung01, DESTINY-Lung02, DESTINY-CRC02, and DESTINY-PanTumor02. Among these patients, 67% were exposed for greater than 6 months and 38% were exposed for greater than 12 months. In this pooled safety population, the most common (≥20%) adverse reactions (including laboratory abnormalities) were decreased white blood cell count (73%), nausea (72%), decreased hemoglobin (67%), decreased neutrophil count (65%), decreased lymphocyte count (60%), fatigue (55%), decreased platelet count (48%), increased aspartate aminotransferase (46%), increased alanine aminotransferase (44%), increased blood alkaline phosphatase (39%), vomiting (38%), alopecia (37%), constipation (32%), decreased blood potassium (32%), decreased appetite (31%), diarrhea (30%), and musculoskeletal pain (24%).
HER2-Positive Locally Advanced or Metastatic Gastric Cancer (6.4 mg/kg)
The data described in WARNINGS and PRECAUTIONS reflect exposure to ENHERTU 6.4 mg/kg intravenously every 3 weeks in 125 patients in DESTINY-Gastric01.
HER2-Positive Metastatic Breast Cancer
DESTINY-Breast03
The safety of ENHERTU was evaluated in 257 patients with unresectable or metastatic HER2-positive breast cancer who received at least one dose of ENHERTU 5.4 mg/kg in DESTINY-Breast03 [see Clinical Studies (14.1)]. ENHERTU was administered by intravenous infusion once every three weeks. The median duration of treatment was 14 months (range: 0.7 to 30) for patients who received ENHERTU and 7 months (range: 0.7 to 25) for patients who received ado-trastuzumab emtansine.
Serious adverse reactions occurred in 19% of patients receiving ENHERTU. Serious adverse reactions in >1% of patients who received ENHERTU were vomiting, interstitial lung disease, pneumonia, pyrexia, and urinary tract infection. Fatalities due to adverse reactions occurred in 0.8% of patients including COVID-19 and sudden death (one patient each).
ENHERTU was permanently discontinued in 14% of patients, of which ILD/pneumonitis accounted for 8%. Dose interruptions due to adverse reactions occurred in 44% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose interruption were neutropenia, leukopenia, anemia, thrombocytopenia, pneumonia, nausea, fatigue, and ILD/pneumonitis. Dose reductions occurred in 21% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose reduction were nausea, neutropenia, and fatigue.
The most common (≥20%) adverse reactions, including laboratory abnormalities, were nausea, decreased white blood cell count, decreased neutrophil count, increased aspartate aminotransferase, decreased hemoglobin, decreased lymphocyte count, increased alanine aminotransferase, decreased platelet count, fatigue, vomiting, increased blood alkaline phosphatase, alopecia, decreased blood potassium, constipation, musculoskeletal pain, diarrhea, decreased appetite, headache, respiratory infection, abdominal pain, increased blood bilirubin, and stomatitis.
Tables 3 and 4 summarize common adverse reactions and laboratory abnormalities observed in DESTINY-Breast03.
Table 3: Common Adverse Reactions (≥10% All Grades or ≥2% Grades 3-4) in Patients Treated with ENHERTU in DESTINY-Breast03 Adverse Reactions ENHERTU
5.4 mg/kg
N=257Ado-trastuzumab emtansine
3.6 mg/kg
N=261All Grades
%Grades 3-4
%All Grades
%Grades 3-4
%Events were graded using NCI CTCAE version 5.0. - *
- Including abdominal pain, abdominal discomfort, lower abdominal pain, and upper abdominal pain
- †
- Including stomatitis, aphthous ulcer, mouth ulceration, oral mucosa erosion, and oral mucosal eruption
- ‡
- Including fatigue, asthenia, malaise, and lethargy
- §
- This Grade 3 event was reported by the investigator. Per NCI CTCAE v.5.0, the highest NCI CTCAE grade for alopecia is Grade 2.
- ¶
- Including back pain, myalgia, pain in extremity, musculoskeletal pain, muscle spasms, bone pain, neck pain, musculoskeletal chest pain, and limb discomfort
- #
- Including respiratory tract infection, lower and upper respiratory tract infection, pneumonia, influenza, influenza-like illness, viral upper respiratory infection, bronchitis, and respiratory syncytial virus infection
- Þ
- Interstitial lung disease includes events that were adjudicated as drug-induced ILD for ENHERTU: pneumonitis, interstitial lung disease, organizing pneumonia, pneumonia, and pulmonary mass. For ado-trastuzumab emtansine: pneumonitis, interstitial lung disease, organizing pneumonia, and pulmonary embolism.
- ß
- Including headache and migraine
- à
- Including peripheral neuropathy, peripheral sensory neuropathy, and paresthesia
Gastrointestinal Disorders Nausea 76 7 30 0.4 Vomiting 49 1.6 10 0.8 Constipation 34 0 20 0 Diarrhea 29 1.2 7 0.4 Abdominal pain* 21 0.8 8 0.4 Stomatitis† 20 0.8 5 0 Dyspepsia 11 0 6 0 General Disorders and Administration Site Conditions Fatigue‡ 49 6 35 0.8 Skin and Subcutaneous Tissue Disorders Alopecia§ 37 0.4 3.1 0 Musculoskeletal and Connective Tissue Disorders Musculoskeletal pain¶ 31 1.2 25 0.4 Metabolism and Nutrition Disorders Decreased appetite 29 1.6 17 0.4 Investigations Decreased weight 17 1.2 6 0.4 Respiratory, Thoracic, and Mediastinal Disorders Respiratory infection# 22 0.8 12 1.1 Epistaxis 11 0 16 0.4 Cough 11 0.4 10 0 Interstitial lung diseaseÞ 11 0.8 1.9 0 Nervous System Disorders Headacheß 22 0.4 16 0 Peripheral neuropathyà 13 0.4 14 0.4 Dizziness 13 0.4 8 0 Other clinically relevant adverse reactions reported in less than 10% of patients in the ENHERTU-treated group were:
- Respiratory, Thoracic, and Mediastinal Disorders: dyspnea (8%)
- Skin and Subcutaneous Tissue Disorders: pruritus (8%) and skin hyperpigmentation (6%) [including skin hyperpigmentation, skin discoloration, and pigmentation disorder]
- Nervous System Disorders: dysgeusia (6%)
- Metabolism and Nutrition Disorders: dehydration (4.3%)
- Eye Disorders: blurred vision (3.5%)
- Cardiac Disorders: asymptomatic left ventricular ejection fraction decrease (2.7%) [see Warnings and Precautions (5.3)]
- Injury, Poisoning, and Procedural Complications: infusion-related reactions (2.3%) [including hypersensitivity and infusion-related reactions]
- Blood and Lymphatic System Disorders: febrile neutropenia (0.8%)
Table 4: Selected Laboratory Abnormalities in Patients in DESTINY-Breast03 Laboratory Parameter ENHERTU
5.4 mg/kg
N=257Ado-trastuzumab emtansine
3.6 mg/kg
N=261All Grades
%Grades 3-4
%All Grades
%Grades 3-4
%Percentages were calculated using patients with worsening laboratory values from baseline and the number of patients with both baseline and post-treatment measurements as the denominator.
Frequencies were based on NCI CTCAE v.5.0 grade-derived laboratory abnormalities.Hematology Decreased white blood cell count 74 8 24 0.8 Decreased neutrophil count 70 18 30 2.3 Decreased hemoglobin 64 7 38 6 Decreased lymphocyte count 55 14 23 3.9 Decreased platelet count 52 7 79 24 Chemistry Increased aspartate aminotransferase 67 0.8 83 5 Increased alanine aminotransferase 53 1.6 67 6 Increased blood alkaline phosphatase 49 0.8 46 0.8 Decreased blood potassium 35 4.7 39 1.5 Increased blood bilirubin 20 0 14 0 Increased blood creatinine 16 0.8 8 0.4 DESTINY-Breast02
The safety of ENHERTU was evaluated in 404 patients with unresectable or metastatic HER2-positive breast cancer who received at least one dose of ENHERTU 5.4 mg/kg in DESTINY-Breast02 [see Clinical Studies (14.1)]. ENHERTU was administered by intravenous infusion once every three weeks. The median duration of treatment was 11 months (range: 0.7 to 45) for patients who received ENHERTU.
Serious adverse reactions occurred in 26% of patients receiving ENHERTU. Serious adverse reactions in >1% of patients who received ENHERTU were COVID-19, ILD, pneumonia, vomiting, fatigue, and nausea. Fatalities due to adverse reactions occurred in 2.5% of patients including pneumonitis (2 patients), acute myeloid leukemia, brain edema, COVID-19, hemorrhage, hepatitis B, malignant pleural effusion, pneumonia, and vasogenic cerebral edema (one patient each).
ENHERTU was permanently discontinued in 20% of patients, of which ILD accounted for 9%. Dose interruptions due to adverse reactions occurred in 45% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose interruption were neutropenia, COVID-19, anemia, fatigue, leukopenia, upper respiratory tract infection, and thrombocytopenia. Dose reductions occurred in 25% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose reduction were fatigue, nausea, neutropenia, and vomiting.
The most common (≥20%) adverse reactions, including laboratory abnormalities, were nausea, decreased white blood cell count, decreased hemoglobin, decreased neutrophil count, fatigue, decreased lymphocyte count, decreased platelet count, increased alanine aminotransferase, vomiting, increased aspartate aminotransferase, alopecia, increased blood alkaline phosphatase, constipation, decreased appetite, decreased blood potassium, diarrhea, musculoskeletal pain, increased blood bilirubin, abdominal pain, and headache.
Tables 5 and 6 summarize common adverse reactions and laboratory abnormalities observed in DESTINY-Breast02.
Table 5: Common Adverse Reactions (≥10% All Grades or ≥2% Grades 3-4) in Patients Treated with ENHERTU in DESTINY-Breast02 Adverse Reactions ENHERTU
5.4 mg/kg
N=404Treatment of Physician's Choice
N=195All Grades
%Grades 3-4
%All Grades
%Grades 3-4
%Events were graded using NCI CTCAE version 5.0. - *
- Including abdominal discomfort, abdominal pain, upper abdominal pain, lower abdominal pain, and gastrointestinal pain
- †
- Including aphthous ulcer, mouth ulceration, and stomatitis
- ‡
- Including asthenia, fatigue, lethargy, and malaise
- §
- Including back pain, bone pain, limb discomfort, musculoskeletal chest pain, musculoskeletal pain, muscle spasms, myalgia, neck pain, and pain in extremity
- ¶
- Including headache and migraine
- #
- Interstitial lung disease includes events that were adjudicated as drug-induced ILD for ENHERTU: pneumonitis, interstitial lung disease, idiopathic interstitial pneumonia, lung disorder, pulmonary toxicity, and pneumonia.
Gastrointestinal Disorders Nausea 73 7 37 2.6 Vomiting 38 3.7 13 1 Constipation 35 0.3 11 0.5 Diarrhea 27 2.7 54 7 Abdominal pain* 22 1 20 2.1 Dyspepsia 12 0 9 0 Stomatitis† 12 1 21 1 General Disorders and Administration Site Conditions Fatigue‡ 62 9 37 1 Skin and Subcutaneous Tissue Disorders Alopecia 37 0.3 4.1 0 Metabolism and Nutrition Disorders Decreased appetite 31 1.7 18 0.5 Musculoskeletal and Connective Tissue Disorders Musculoskeletal pain§ 25 0.7 18 0.5 Nervous System Disorders Headache¶ 20 0.3 6 0 Investigations Decreased weight 18 0.3 3.6 0 Respiratory, Thoracic, and Mediastinal Disorders Cough 13 0 10 0 Interstitial lung disease# 10 0.7 0.5 0.5 Other clinically relevant adverse reactions reported in less than 10% of patients in the ENHERTU-treated group were:
- Respiratory, Thoracic, and Mediastinal Disorders: dyspnea (8%) and epistaxis (8%)
- Skin and Subcutaneous Tissue Disorders: rash (8%) [including rash, pustular rash, maculo-papular rash, and pruritic rash], pruritus (5%), skin hyperpigmentation (5%) [including skin hyperpigmentation and pigmentation disorder]
- Nervous System Disorders: dizziness (8%) and dysgeusia (8%)
- Cardiac Disorders: asymptomatic left ventricular ejection fraction decrease (4.2%) [see Warnings and Precautions (5.3)]
- Eye Disorders: dry eye (6%) and blurred vision [including blurred vision and visual impairment] (3%)
- Metabolism and Nutrition Disorders: dehydration (2.7%)
- Injury, Poisoning, and Procedural Complications: infusion-related reactions (1.2%)
- Blood and Lymphatic System Disorders: febrile neutropenia (0.3%)
Table 6: Selected Laboratory Abnormalities in Patients in DESTINY-Breast02 Laboratory Parameter ENHERTU
5.4 mg/kg
N=404Treatment of Physician's Choice
N=195All Grades
%Grades 3-4
%All Grades
%Grades 3-4
%Percentages were calculated using patients with worsening laboratory values from baseline and the number of patients with both baseline and post-treatment measurements as the denominator.
Frequencies were based on NCI CTCAE v.5.0 grade-derived laboratory abnormalities.Hematology Decreased white blood cell count 70 12 42 3.2 Decreased hemoglobin 67 9 54 3.2 Decreased neutrophil count 64 16 34 4.7 Decreased lymphocyte count 58 17 38 4.7 Decreased platelet count 48 2.7 31 1.6 Chemistry Increased alanine aminotransferase 43 1 32 1.6 Increased aspartate aminotransferase 37 0.7 29 2.1 Increased blood alkaline phosphatase 37 0 17 0 Decreased blood potassium 30 3.7 29 8 Increased blood bilirubin 23 0.3 44 2.1 Increased blood creatinine 7 0.3 13 0 DESTINY-Breast01 and Study DS8201-A-J101
The safety of ENHERTU was evaluated in a pooled analysis of 234 patients with unresectable or metastatic HER2-positive breast cancer who received at least one dose of ENHERTU 5.4 mg/kg in DESTINY-Breast01 and Study DS8201-A-J101 (NCT02564900) [see Clinical Studies (14.1)]. ENHERTU was administered by intravenous infusion once every three weeks. The median duration of treatment was 7 months (range: 0.7 to 31).
In the pooled 234 patients, the median age was 56 years (range: 28-96), 74% of patients were <65 years, 99.6% of patients were female, and the majority were White (51%) or Asian (42%). Patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 (58%) or 1 (42%) at baseline. Ninety-four percent had visceral disease, 31% had bone metastases, and 13% had brain metastases.
Serious adverse reactions occurred in 20% of patients receiving ENHERTU. Serious adverse reactions in >1% of patients who received ENHERTU were interstitial lung disease, pneumonia, vomiting, nausea, cellulitis, decreased blood potassium, and intestinal obstruction. Fatalities due to adverse reactions occurred in 4.3% of patients including interstitial lung disease (2.6%), and the following events occurred in one patient each (0.4%): acute hepatic failure/acute kidney injury, general physical health deterioration, pneumonia, and hemorrhagic shock.
ENHERTU was permanently discontinued in 9% of patients, of which ILD accounted for 6%.
Dose interruptions due to adverse reactions occurred in 33% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose interruption were neutropenia, anemia, thrombocytopenia, leukopenia, upper respiratory tract infection, fatigue, nausea, and ILD.
Dose reductions occurred in 18% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose reduction were fatigue, nausea, and neutropenia.
The most common (≥20%) adverse reactions, including laboratory abnormalities, were nausea, decreased white blood cell count, decreased hemoglobin, decreased neutrophil count, fatigue, vomiting, alopecia, increased aspartate aminotransferase, increased alanine aminotransferase, decreased platelet count, constipation, decreased appetite, diarrhea, decreased blood potassium, and cough.
Tables 7 and 8 summarize common adverse reactions and laboratory abnormalities observed in ENHERTU-treated patients in DESTINY-Breast01 and Study DS8201-A-J101.
Table 7: Common Adverse Reactions (≥10% All Grades or ≥2% Grades 3 or 4) in Patients in DESTINY-Breast01 and Study DS8201-A-J101 Adverse Reactions ENHERTU 5.4 mg/kg
N=234All Grades
%Grades 3 or 4
%Events were graded using NCI CTCAE version 4.03. - *
- Including abdominal discomfort, gastrointestinal pain, abdominal pain, lower abdominal pain, and upper abdominal pain
- †
- Including stomatitis, aphthous ulcer, mouth ulceration, oral mucosa erosion, and oral mucosa blistering. One Grade 1 event of aphthous ulcer was not included in the summary of grouped term stomatitis (from DESTINY-Breast01).
- ‡
- Including fatigue and asthenia
- §
- This Grade 3 event was reported by the investigator. Per NCI CTCAE v.4.03, the highest NCI CTCAE grade for alopecia is Grade 2.
- ¶
- Including rash, pustular rash, and maculo-papular rash
- #
- Interstitial lung disease includes events that were adjudicated as drug-induced ILD: pneumonitis, interstitial lung disease, respiratory failure, organizing pneumonia, acute respiratory failure, lung infiltration, lymphangitis, and alveolitis.
- Þ
- All events had fatal outcomes (n=6).
- ß
- Including headache, sinus headache, and migraine
- à
- Including influenza, influenza-like illness, and upper respiratory tract infection
- è
- This Grade 4 event was reported by the investigator. Per NCI CTCAE v.4.03, the highest NCI CTCAE grade for dry eye is Grade 3.
Gastrointestinal Disorders Nausea 79 7 Vomiting 47 3.8 Constipation 35 0.9 Diarrhea 29 1.7 Abdominal pain* 19 1.3 Stomatitis† 14 0.9 Dyspepsia 12 0 General Disorders and Administration Site Conditions Fatigue‡ 59 6 Skin and Subcutaneous Tissue Disorders Alopecia 46 0.4§ Rash¶ 10 0 Metabolism and Nutrition Disorders Decreased appetite 32 1.3 Respiratory, Thoracic, and Mediastinal Disorders Cough 20 0 Dyspnea 13 1.3 Epistaxis 13 0 Interstitial lung disease# 9 2.6Þ Nervous System Disorders Headacheß 19 0 Dizziness 10 0 Infections and Infestations Upper respiratory tract infectionà 15 0 Eye Disorders Dry eye 11 0.4è Other clinically relevant adverse reactions reported in less than 10% of patients were:
- Injury, Poisoning, and Procedural Complications: infusion-related reactions (2.6%)
- Blood and Lymphatic System Disorders: febrile neutropenia (1.7%)
Table 8: Selected Laboratory Abnormalities in Patients with Unresectable or Metastatic HER2-positive Breast Cancer Treated with ENHERTU in DESTINY-Breast01 and Study DS8201-A-J101 Laboratory Parameter ENHERTU 5.4 mg/kg
N=234All Grades
%Grades 3 or 4
%Percentages were calculated using patients with worsening laboratory values from baseline and the number of patients with both baseline and post-treatment measurements as the denominator. Frequencies were based on NCI CTCAE v.4.03 grade-derived laboratory abnormalities. Hematology Decreased white blood cell count 70 7 Decreased hemoglobin 70 7 Decreased neutrophil count 62 16 Decreased platelet count 37 3.4 Chemistry Increased aspartate aminotransferase 41 0.9 Increased alanine aminotransferase 38 0.4 Decreased blood potassium 26 3 HER2-Low and HER2-Ultralow Metastatic Breast Cancer
DESTINY-Breast06
The safety of ENHERTU was evaluated in 434 patients with unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) or HER2-ultralow (IHC 0 with membrane staining) breast cancer who received ENHERTU 5.4 mg/kg in DESTINY-Breast06 [see Clinical Studies (14.2)]. ENHERTU was administered by intravenous infusion once every three weeks. The median duration of treatment was 11 months (range: 0.4 to 39.6) for patients who received ENHERTU.
Serious adverse reactions occurred in 20% of patients receiving ENHERTU. Serious adverse reactions in >1% of patients who received ENHERTU were interstitial lung disease (ILD)/pneumonitis, COVID-19, febrile neutropenia, and hypokalemia. Fatalities due to adverse reactions occurred in 2.8% of patients including ILD (0.7%); sepsis (0.5%); and COVID-19 pneumonia, bacterial meningoencephalitis, neutropenic sepsis, peritonitis, cerebrovascular accident, general physical health deterioration (0.2% each).
ENHERTU was permanently discontinued in 14% of patients. The most frequent adverse reactions (>2%) associated with permanent discontinuation was ILD/pneumonitis.
Dose interruptions due to adverse reactions occurred in 48% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose interruption were COVID-19, decreased neutrophil count, anemia, pyrexia, pneumonia, decreased white blood cell count, and ILD.
Dose reductions occurred in 25% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose reduction were nausea, fatigue, decreased platelet count, and decreased neutrophil count.
The most common (≥20%) adverse reactions, including laboratory abnormalities, were decreased white blood cell count, decreased neutrophil count, nausea, decreased hemoglobin, decreased lymphocyte count, fatigue, decreased platelet count, alopecia, increased alanine aminotransferase, increased blood alkaline phosphatase, increased aspartate aminotransferase, decreased blood potassium, diarrhea, vomiting, constipation, decreased appetite, COVID-19, and musculoskeletal pain.
Tables 9 and 10 summarize common adverse reactions and laboratory abnormalities observed in DESTINY-Breast06.
Table 9: Common Adverse Reactions (≥10% All Grades or ≥2% Grades 3 or 4) in Patients Treated with ENHERTU in DESTINY-Breast06 Adverse Reactions ENHERTU
5.4 mg/kgChemotherapy N=434 N=417 All Grades
%Grades 3 or 4
%All Grades
%Grades 3 or 4
%Events were graded using NCI CTCAE version 5.0. - *
- Including abdominal discomfort, abdominal pain, lower abdominal pain, upper abdominal pain, and gastrointestinal pain
- †
- Including stomatitis, aphthous ulcer, mouth ulceration, oral mucosa erosion, oral mucosal blistering, and oral mucosal eruption
- ‡
- Including fatigue, asthenia, malaise, and lethargy
- §
- Including dermatitis, dermatitis allergic, dermatitis contact, eczema, palmar-plantar erythrodysesthesia syndrome, rash, rash erythematous, rash macular, rash maculo-papular, rash papular, rash pruritic, rash pustular
- ¶
- Including COVID-19, COVID-19 pneumonia
- #
- Including influenza, influenza-like illness, upper respiratory tract infection, nasopharyngitis, pharyngitis, sinusitis, rhinitis, laryngitis
- Þ
- Including back pain, myalgia, pain in extremity, musculoskeletal pain, muscle spasms, bone pain, neck pain, musculoskeletal chest pain, and limb discomfort
- ß
- Including migraine, headache, and sinus headache
- à
- Including bronchiectasis, interstitial lung disease, lower respiratory tract infection, pneumonia, pneumonia bacterial, pneumonitis, and pulmonary toxicity
Gastrointestinal Disorders Nausea 70 2.1 30 0.5 Diarrhea 34 2.3 27 2.6 Vomiting 34 1.4 12 0.2 Constipation 32 0.7 15 0.5 Abdominal pain* 20 0.5 14 0.2 Stomatitis† 15 0 11 0.5 Dyspepsia 12 0 4.8 0 General Disorders and Administration Site Conditions Fatigue‡ 53 4.4 40 2.4 Pyrexia 12 0.2 7 0 Skin and Subcutaneous Tissue Disorders Alopecia 48 0 21 0.5 Rash§ 12 0.2 43 8 Metabolism and Nutrition Disorders Decreased appetite 26 1.4 12 0.5 Infections and Infestations COVID-19¶ 26 0.9 13 1 Upper respiratory tract infection# 19 0 9 0 Musculoskeletal and Connective Tissue Disorders Musculoskeletal painÞ 24 0.5 23 1.9 Nervous System Disorders Headacheß 18 0.5 10 0 Dysgeusia 12 0.2 6 0 Respiratory, Thoracic, and Mediastinal Disorders Cough 16 0 9 0 Interstitial lung diseaseà 11 0.7 0.2 0 Epistaxis 10 0 3.6 0.2 Other clinically relevant adverse reactions reported in less than 10% of patients in the ENHERTU-treated group were:
- Nervous System Disorders: dizziness (9%)
- General Disorders and Administration Site Conditions: peripheral edema (8%)
- Investigations: decreased weight (7%)
- Eye Disorders: dry eye (7%), and blurred vision (5%)
- Respiratory, Thoracic, and Mediastinal Disorders: dyspnea (6%)
- Gastrointestinal Disorders: abdominal distension (4.8%), flatulence (2.3%), and gastritis (0.7%)
- Infections and Infestations: pneumonia (4.6%)
- Skin and Subcutaneous Tissue Disorders: pruritus (3.9%), and skin hyperpigmentation (0.9%)
- Metabolism and Nutrition Disorders: dehydration (1.6%)
- Blood and lymphatic system disorders: febrile neutropenia (1.2%)
- Injury, Poisoning, and Procedural Complications: infusion related reaction (1.2%)
Table 10: Selected Laboratory Abnormalities in Patients in DESTINY-Breast06 Laboratory Parameter ENHERTU
5.4 mg/kgChemotherapy N=434 N=417 All Grades
%Grades 3 or 4
%All Grades
%Grades 3 or 4
%Percentages were calculated using patients with worsening laboratory values from baseline and the number of patients with both baseline and post-treatment measurements as the denominator. Frequencies were based on NCI CTCAE v.5.0 grade-derived laboratory abnormalities. Hematology Decreased white blood cell count 86 13 71 11 Decreased neutrophil count 75 27 53 20 Decreased hemoglobin 69 9 58 5 Decreased lymphocyte count 66 19 46 8 Decreased platelet count 48 6 25 1 Chemistry Increased alanine aminotransferase 44 3.2 30 0.7 Increased blood alkaline phosphatase 43 0.2 22 0.2 Increased aspartate aminotransferase 41 2.6 27 1.2 Decreased blood potassium 35 8 15 2.9 Increased blood bilirubin 16 1.9 23 1.5 Increased blood creatinine 10 1.9 8 1 DESTINY-Breast04
The safety of ENHERTU was evaluated in 371 patients with unresectable or metastatic HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer who received ENHERTU 5.4 mg/kg in DESTINY-Breast04 [see Clinical Studies (14.2)]. ENHERTU was administered by intravenous infusion once every three weeks. The median duration of treatment was 8 months (range: 0.2 to 33) for patients who received ENHERTU.
Serious adverse reactions occurred in 28% of patients receiving ENHERTU. Serious adverse reactions in >1% of patients who received ENHERTU were ILD/pneumonitis, pneumonia, dyspnea, musculoskeletal pain, sepsis, anemia, febrile neutropenia, hypercalcemia, nausea, pyrexia, and vomiting. Fatalities due to adverse reactions occurred in 4.0% of patients including ILD/pneumonitis (3 patients); sepsis (2 patients); and ischemic colitis, disseminated intravascular coagulation, dyspnea, febrile neutropenia, general physical health deterioration, pleural effusion, and respiratory failure (1 patient each).
ENHERTU was permanently discontinued in 16% of patients, of which ILD/pneumonitis accounted for 8%. Dose interruptions due to adverse reactions occurred in 39% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose interruption were neutropenia, fatigue, anemia, leukopenia, COVID-19, ILD/pneumonitis, increased transaminases, and hyperbilirubinemia. Dose reductions occurred in 23% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose reduction were fatigue, nausea, thrombocytopenia, and neutropenia.
The most common (≥20%) adverse reactions, including laboratory abnormalities, were nausea, decreased white blood cell count, decreased hemoglobin, decreased neutrophil count, decreased lymphocyte count, fatigue, decreased platelet count, alopecia, vomiting, increased aspartate aminotransferase, increased alanine aminotransferase, constipation, increased blood alkaline phosphatase, decreased appetite, musculoskeletal pain, diarrhea, and decreased blood potassium.
Tables 11 and 12 summarize common adverse reactions and laboratory abnormalities observed in DESTINY-Breast04.
Table 11: Common Adverse Reactions (≥10% All Grades or ≥2% Grades 3 or 4) in Patients Treated with ENHERTU in DESTINY-Breast04 Adverse Reactions ENHERTU
5.4 mg/kgChemotherapy N=371 N=172 All Grades
%Grades 3 or 4
%All Grades
%Grades 3 or 4
%Events were graded using NCI CTCAE version 5.0. - *
- Including abdominal pain, abdominal discomfort, lower abdominal pain, and upper abdominal pain
- †
- Including stomatitis, aphthous ulcer, mouth ulceration, and pharyngeal inflammation
- ‡
- Including fatigue, asthenia, and malaise
- §
- Including rash, pustular rash, pruritic rash, maculo-papular rash, palmar-plantar erythrodysesthesia syndrome, papular rash, macular rash, eczema, erythema multiforme, dermatitis, urticarial dermatitis, drug eruption, and dermatitis bullous
- ¶
- Including back pain, myalgia, pain in extremity, musculoskeletal pain, bone pain, musculoskeletal chest pain, arthralgia, noncardiac chest pain, musculoskeletal stiffness, arthritis, spinal pain, and neck pain
- #
- Including esophageal varices, hemorrhage, hemorrhoidal hemorrhage, epistaxis, hematuria, conjunctival hemorrhage, vaginal hemorrhage, gingival bleeding, genital hemorrhage, eye hemorrhage, hemoptysis, hemorrhagic cystitis, pharyngeal hemorrhage, rectal hemorrhage, upper gastrointestinal hemorrhage, and esophageal hemorrhage
- Þ
- Including headache and migraine
- ß
- Including peripheral neuropathy, peripheral sensory neuropathy, peripheral motor neuropathy, polyneuropathy, paresthesia, hypoesthesia, dysesthesia, and neuralgia
- à
- Including dizziness, postural dizziness, and vertigo
- è
- Including upper respiratory tract infection, influenza, influenza-like illness, nasopharyngitis, pharyngitis, sinusitis, and rhinitis
- ð
- Interstitial lung disease includes events that were adjudicated as drug-induced ILD for ENHERTU: interstitial lung disease, pneumonitis, organizing pneumonia, pneumonia, and radiation pneumonitis.
Gastrointestinal Disorders Nausea 76 4.6 30 0 Vomiting 40 1.6 13 0 Constipation 34 0.8 22 0 Diarrhea 27 1.3 22 1.7 Abdominal pain* 18 0.5 13 0 Stomatitis† 13 0.3 12 0.6 General Disorders and Administration Site Conditions Fatigue‡ 54 9 48 4.7 Pyrexia 12 0.3 13 0 Skin and Subcutaneous Tissue Disorders Alopecia 40 0 33 0 Rash§ 13 0 23 4.7 Metabolism and Nutrition Disorders Decreased appetite 32 2.4 19 1.2 Musculoskeletal and Connective Tissue Disorders Musculoskeletal pain¶ 32 1.3 31 0.6 Investigations Decreased weight 16 0.3 8 0 Vascular Disorders Hemorrhage# 16 0 3.5 0 Nervous System Disorders HeadacheÞ 15 0.3 6 0 Peripheral neuropathyß 13 0 29 5 Dizzinessà 11 0.5 6 0 Infections and Infestations Upper respiratory tract infectionè 14 0.3 5 0 Respiratory, Thoracic and Mediastinal Disorders Interstitial lung diseaseð 12 1.3 0.6 0 Dyspnea 10 1.3 9 1.2 Other clinically relevant adverse reactions reported in less than 10% of patients treated with ENHERTU:
- Nervous System Disorders: dysgeusia (10%)
- Respiratory, Thoracic and Mediastinal Disorders: cough (10%)
- Gastrointestinal Disorders: abdominal distension (5%), gastritis (2.7%), flatulence (2.4%)
- Eye Disorders: blurred vision (4.9%) [including blurred vision and visual impairment]
- Skin and Subcutaneous Tissue Disorders: pruritus (3.2%) and skin hyperpigmentation (2.7%) [including skin hyperpigmentation, skin discoloration, and pigmentation disorder]
- Metabolism and Nutrition Disorders: dehydration (1.9%)
- Blood and Lymphatic System Disorders: febrile neutropenia (1.1%)
- Injury, Poisoning, and Procedural Complications: infusion-related reactions (0.5%) [including injection-site reaction and chills]
Table 12: Selected Laboratory Abnormalities in Patients in DESTINY-Breast04 Laboratory Parameter ENHERTU
5.4 mg/kgChemotherapy N=371 N=172 All Grades
%Grades 3 or 4
%All Grades
%Grades 3 or 4
%Percentages were calculated using patients with worsening laboratory values from baseline and the number of patients with both baseline and post-treatment measurements as the denominator.
Frequencies were based on NCI CTCAE v.5.0 grade-derived laboratory abnormalities.Hematology Decreased white blood cell count 70 9 78 25 Decreased hemoglobin 64 8 53 6 Decreased neutrophil count 64 14 73 38 Decreased lymphocyte count 55 18 40 11 Decreased platelet count 44 6 21 0.6 Chemistry Increased aspartate aminotransferase 38 2.2 38 4.1 Increased alanine aminotransferase 36 0.8 38 4.1 Increased blood alkaline phosphatase 34 0.3 24 0 Decreased blood potassium 25 3.3 17 1.2 Increased blood bilirubin 16 2.7 15 0.6 Increased blood creatinine 15 1.1 9 0.6 HER2-Mutant Unresectable or Metastatic NSCLC
DESTINY-Lung02 evaluated two dose levels (5.4 mg/kg [n=101] and 6.4 mg/kg [n=50]); however, only the results for the recommended dose of 5.4 mg/kg intravenously every 3 weeks are described below due to increased toxicity observed with the higher dose in patients with NSCLC, including ILD/pneumonitis.
The safety of ENHERTU was evaluated in 101 patients in DESTINY-Lung02 [see Clinical Studies (14.3)]. Patients received ENHERTU 5.4 mg/kg intravenously once every three weeks until disease progression or unacceptable toxicity. Nineteen percent of patients were exposed for greater than 6 months. The median age was 59 years (range 30 to 83); 64% were female; 23% were White, 64% were Asian, and 14% were other races.
Serious adverse reactions occurred in 30% of patients receiving ENHERTU. Serious adverse reactions in >1% of patients who received ENHERTU were ILD/pneumonitis, thrombocytopenia, dyspnea, nausea, pleural effusion, and increased troponin I. Fatality occurred in 1 patient with suspected ILD/pneumonitis (1%).
ENHERTU was permanently discontinued due to an adverse reaction in 8% of patients. Adverse reactions which resulted in permanent discontinuation of ENHERTU were ILD/pneumonitis, diarrhea, decreased blood potassium, hypomagnesemia, myocarditis, and vomiting.
Dose interruptions of ENHERTU due to adverse reactions occurred in 23% of patients. Adverse reactions which required dose interruption (>2%) included neutropenia and ILD/pneumonitis.
Dose reductions due to an adverse reaction occurred in 11% of patients.
The most common (≥20%) adverse reactions, including laboratory abnormalities, were nausea, decreased white blood cell count, decreased hemoglobin, decreased neutrophil count, decreased lymphocyte count, decreased platelet count, decreased albumin, increased aspartate aminotransferase, increased alanine aminotransferase, fatigue, constipation, decreased appetite, vomiting, increased alkaline phosphatase, and alopecia.
Tables 13 and 14 summarize common adverse reactions and laboratory abnormalities observed in DESTINY-Lung02.
Table 13: Common Adverse Reactions (≥10% All Grades or ≥2% Grades 3 or 4) in Patients with Unresectable or Metastatic HER2-Mutant NSCLC in DESTINY-Lung02 Adverse Reactions ENHERTU 5.4 mg/kg
N=101All Grades
%Grades 3 or 4
%Events were graded using NCI CTCAE version 5.0. Gastrointestinal Disorders Nausea 61 3 Constipation 31 1 Vomiting* 26 2 Diarrhea 19 1 Stomatitis† 12 0 General Disorders and Administration Site Conditions Fatigue‡ 32 4 Metabolism and Nutrition Disorders Decreased appetite 30 1 Skin and Subcutaneous Tissue Disorders Alopecia 21 0 Musculoskeletal and Connective Tissue Disorders Musculoskeletal pain§ 15 1 Other clinically relevant adverse reactions reported in less than 10% of patients were:
- Respiratory, Thoracic and Mediastinal Disorders: interstitial lung disease (6%) [including interstitial lung disease that was adjudicated as drug-induced ILD including pneumonitis, interstitial lung disease, pulmonary toxicity, and respiratory failure], dyspnea (5%), and epistaxis (3%)
- Gastrointestinal Disorders: abdominal pain (9%) [including abdominal discomfort, abdominal pain, and upper abdominal pain]
- Skin and Subcutaneous Disorders: rash (3%) [including rash and maculo-papular rash]
- Infections and Infestations: upper respiratory tract infection (4%) [including upper respiratory tract infection, pharyngitis, and laryngitis]
- Nervous System Disorders: headache (4%) [including headache and migraine]
Table 14: Select Laboratory Abnormalities in Patients with Unresectable or Metastatic HER2-Mutant NSCLC in DESTINY-Lung02 Laboratory Parameter ENHERTU 5.4 mg/kg
N=101*All Grades†
%Grades 3 or 4†
%- *
- Percentages were calculated using patients with worsening laboratory values from baseline and the number of patients with both baseline and post-treatment measurements as the denominator.
- †
- Frequencies were based on NCI CTCAE v.5.0 grade-derived laboratory abnormalities.
- ‡
- The denominator used to calculate the rate varied from 98 to 99 based on the number of patients with a baseline value and at least one post-treatment value.
Hematology‡ Decreased white blood cell count 60 4 Decreased hemoglobin 58 10 Decreased neutrophil count 52 12 Decreased lymphocyte count 43 16 Decreased platelet count 40 4 Chemistry Decreased albumin 39 0 Increased aspartate aminotransferase 35 1 Increased alanine aminotransferase 34 2 Increased alkaline phosphatase 22 0 Decreased blood potassium 17 2 HER2-Positive Locally Advanced or Metastatic Gastric Cancer
The safety of ENHERTU was evaluated in 187 patients with locally advanced or metastatic HER2-positive gastric or GEJ adenocarcinoma in DESTINY-Gastric01 [see Clinical Studies (14.4)]. Patients intravenously received at least one dose of either ENHERTU (N=125) 6.4 mg/kg once every three weeks or either irinotecan (N=55) 150 mg/m2 biweekly or paclitaxel (N=7) 80 mg/m2 weekly for 3 weeks. The median duration of treatment was 4.6 months (range: 0.7 to 22.3) in the ENHERTU group and 2.8 months (range: 0.5 to 13.1) in the irinotecan/paclitaxel group.
Serious adverse reactions occurred in 44% of patients receiving ENHERTU 6.4 mg/kg. Serious adverse reactions in >2% of patients who received ENHERTU were decreased appetite, ILD, anemia, dehydration, pneumonia, cholestatic jaundice, pyrexia, and tumor hemorrhage. Fatalities due to adverse reactions occurred in 2.4% of patients: disseminated intravascular coagulation, large intestine perforation, and pneumonia occurred in one patient each (0.8%).
ENHERTU was permanently discontinued in 15% of patients, of which ILD accounted for 6%.
Dose interruptions due to adverse reactions occurred in 62% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose interruption were neutropenia, anemia, decreased appetite, leukopenia, fatigue, thrombocytopenia, ILD, pneumonia, lymphopenia, upper respiratory tract infection, diarrhea, and decreased blood potassium.
Dose reductions occurred in 32% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose reduction were neutropenia, decreased appetite, fatigue, nausea, and febrile neutropenia.
The most common (≥20%) adverse reactions, including laboratory abnormalities, were decreased hemoglobin, decreased white blood cell count, decreased neutrophil count, decreased lymphocyte count, decreased platelet count, nausea, decreased appetite, increased aspartate aminotransferase, fatigue, increased blood alkaline phosphatase, increased alanine aminotransferase, diarrhea, decreased blood potassium, vomiting, constipation, increased blood bilirubin, pyrexia, and alopecia.
Tables 15 and 16 summarize adverse reactions and laboratory abnormalities observed in patients receiving ENHERTU 6.4 mg/kg in DESTINY-Gastric01.
Table 15: Adverse Reactions in ≥10% All Grades or ≥2% Grades 3 or 4 of Patients Receiving ENHERTU in DESTINY-Gastric01 ENHERTU 6.4 mg/kg
N=125Irinotecan or Paclitaxel
N=62Adverse Reactions All Grades
%Grades 3 or 4
%All Grades
%Grades 3 or 4
%Events were graded using NCI CTCAE version 4.03. - *
- Including abdominal discomfort, gastrointestinal pain, abdominal pain, lower abdominal pain, and upper abdominal pain
- †
- Including stomatitis, aphthous ulcer, mouth ulceration, oral mucosa erosion, and oral mucosal blistering
- ‡
- Including fatigue, asthenia, and malaise
- §
- Interstitial lung disease includes events that were adjudicated as drug-induced ILD: pneumonitis, interstitial lung disease, respiratory failure, organizing pneumonia, acute respiratory failure, lung infiltration, lymphangitis, and alveolitis.
Gastrointestinal Disorders Nausea 63 4.8 47 1.6 Diarrhea 32 2.4 32 1.6 Vomiting 26 0 8 0 Constipation 24 0 23 0 Abdominal pain* 14 0.8 15 3.2 Stomatitis† 11 1.6 4.8 0 Metabolism and Nutrition Disorders Decreased appetite 60 17 45 13 Dehydration 6 2.4 3.2 1.6 Blood and Lymphatic System Disorders Febrile neutropenia 4.8 4.8 3.2 3.2 General Disorders and Administration Site Conditions Fatigue‡ 55 9 44 4.8 Pyrexia 24 0 16 0 Peripheral edema 10 0 0 0 Skin and Subcutaneous Tissue Disorders Alopecia 22 0 15 0 Respiratory, Thoracic and Mediastinal Disorders Interstitial lung disease§ 10 2.4 0 0 Hepatobiliary Disorders Abnormal hepatic function 8 3.2 1.6 1.6 Other clinically relevant adverse reactions reported in less than 10% of patients were:
- Cardiac Disorders: asymptomatic left ventricular ejection fraction decrease (8%) [see Warnings and Precautions (5.3)]
- Infections and Infestations: pneumonia (6%)
- Injury, Poisoning, and Procedural Complications: infusion-related reactions (1.6%)
Table 16: Selected Laboratory Abnormalities Occurring in Patients Receiving ENHERTU in DESTINY-Gastric01 Laboratory Parameter ENHERTU 6.4 mg/kg
N=125Irinotecan or Paclitaxel
N=62All Grades
%Grades 3 or 4
%All Grades
%Grades 3 or 4
%Percentages were calculated using patients with worsening laboratory values from baseline and the number of patients with both baseline and post-treatment measurements as the denominator.
Frequencies were based on NCI CTCAE v.4.03 grade-derived laboratory abnormalities.Hematology Decreased hemoglobin 75 38 55 23 Decreased white blood cell count 74 29 53 13 Decreased neutrophil count 72 51 45 23 Decreased lymphocyte count 70 28 53 12 Decreased platelet count 68 12 12 5 Chemistry Increased aspartate aminotransferase 58 9 32 8 Increased blood alkaline phosphatase 54 8 34 10 Increased alanine aminotransferase 47 9 17 1.7 Decreased blood potassium 30 4.8 18 8 Increased blood bilirubin 24 7 5 3.4 HER2-Positive (IHC 3+) Unresectable or Metastatic Solid Tumors
The safety of ENHERTU was evaluated in 347 adult patients with unresectable or metastatic HER2-positive (IHC 3+) solid tumors who received ENHERTU 5.4 mg/kg in DESTINY-Breast01, DESTINY-PanTumor02, DESTINY-Lung01, and DESTINY-CRC02 [see Clinical Studies (14.1 and 14.5)]. ENHERTU was administered by intravenous infusion once every three weeks. The median duration of treatment was 8.3 months (range 0.7 to 30.2).
The median age was 60 years (range 23 to 96); 74% were female; 51% were White, 42% were Asian, 2.9% were Black or African American, 3.5% were of Hispanic or Latino ethnicity; and 40% had an ECOG performance status 0 and 41% had an ECOG performance status of 1.
Serious adverse reactions occurred in 34% of patients receiving ENHERTU. Serious adverse reactions in >1% of patients who received ENHERTU were sepsis, pneumonia, vomiting, urinary tract infection, abdominal pain, nausea, pneumonitis, pleural effusion, hemorrhage, COVID-19, fatigue, acute kidney injury, anemia, cellulitis, and dyspnea. Fatalities due to adverse reactions occurred in 6.3% of patients including ILD/pneumonitis (2.3%), cardiac arrest (0.6%), COVID-19 (0.6%), and sepsis (0.6%). The following events occurred in one patient each (0.3%): acute kidney injury, cerebrovascular accident, general physical health deterioration, pneumonia, and hemorrhagic shock.
ENHERTU was permanently discontinued in 15% of patients, of which ILD/pneumonitis accounted for 10%.
Dose interruptions due to adverse reactions occurred in 48% of patients. The most frequent adverse reactions (>2%) associated with dose interruption were decreased neutrophil count, anemia, COVID-19, fatigue, decreased white blood cell count, and ILD/pneumonitis.
Dose reductions occurred in 27% of patients treated with ENHERTU. The most frequent adverse reactions (>2%) associated with dose reduction were fatigue, nausea, decreased neutrophil count, ILD/pneumonitis, and diarrhea.
The most common (≥20%) adverse reactions, including laboratory abnormalities, were decreased white blood cell count, nausea, decreased hemoglobin, decreased neutrophil count, fatigue, decreased lymphocyte count, decreased platelet count, increased aspartate aminotransferase, increased alanine aminotransferase, increased blood alkaline phosphatase, vomiting, decreased appetite, alopecia, diarrhea, decreased blood potassium, constipation, decreased sodium, stomatitis, and upper respiratory tract infection.
Tables 17 and 18 summarize the common adverse reactions and laboratory abnormalities in DESTINY-PanTumor02, DESTINY-Lung01, DESTINY-Breast01, and DESTINY-CRC02.
Table 17: Common Adverse Reactions (≥10% All Grades or ≥2% Grades 3 or 4) in HER2-positive (IHC 3+) Patients Treated with ENHERTU in DESTINY-Breast01, DESTINY-PanTumor02, DESTINY-Lung01, and DESTINY-CRC02 Adverse Reactions ENHERTU 5.4 mg/kg
N= 347All Grades
%Grade 3 or 4
%- *
- Including stomatitis, mucosal inflammation, aphthous ulcer, mouth ulceration, oral mucosa erosion, oral mucosal blistering, oral mucosal eruption, tongue ulceration, cheilitis.
- †
- Including abdominal discomfort, abdominal pain, lower abdominal pain, upper abdominal pain, gastrointestinal pain.
- ‡
- Including fatigue, asthenia, malaise, lethargy.
- §
- Including peripheral edema, edema, localized edema, face edema, skin edema, periorbital edema, eyelid edema
- ¶
- Including rash, pustular rash, maculo-papular rash, papular rash, macular rash, pruritic rash dermatitis acneiform, dermatitis, eczema, palmar-plantar erythrodysesthesia syndrome.
- #
- Including influenza, influenza-like illness, upper respiratory tract infection, nasopharyngitis, pharyngitis, sinusitis, rhinitis, laryngitis.
- Þ
- Including back pain, myalgia, pain in extremity, musculoskeletal pain, muscle spasms, bone pain, neck pain, musculoskeletal chest pain, limb discomfort.
- ß
- Including cough, productive cough, upper-airway cough syndrome
- à
- Interstitial lung disease includes events that were adjudicated as drug-induced ILD: pneumonitis, ILD, organizing pneumonia, respiratory failure, acute respiratory failure, alveolitis, lung opacity, lymphangitis, pneumonia, bacterial pneumonia, pulmonary fibrosis, and radiation pneumonitis. Grade 5 adjudicated drug-induced ILD events were pneumonitis, respiratory failure, acute respiratory failure, lymphangitis, pulmonary fibrosis.
- è
- Including dyspnea, exertional dyspnea
- ð
- Including migraine, headache, sinus headache.
Gastrointestinal Disorders Nausea 69 7 Vomiting 35 3.5 Diarrhea 31 4.3 Constipation 28 0.6 Stomatitis* 20 0.9 Abdominal pain† 18 2 Dyspepsia 12 0.3 General Disorders and Administration Site Conditions Fatigue‡ 59 10 Pyrexia 11 0 Edema§ 11 0.6 Metabolism and Nutrition Disorders Decreased appetite 34 2.6 Skin and Subcutaneous Tissue Disorders Alopecia 34 0.3 Rash¶ 13 0.6 Infections and Infestations Upper respiratory tract infection# 20 0 Pneumonia 6 2.3 Musculoskeletal and Connective Tissue Disorders Musculoskeletal painÞ 19 0.3 Respiratory, Thoracic and Mediastinal Disorders Coughß 18 0 Interstitial lung diseaseà 16 0.6 Dyspneaè 12 1.7 Nervous System Disorders Headacheð 15 0 Investigations Decreased weight 10 0.3 Other clinically relevant adverse reactions reported in less than 10% of patients were:
- Respiratory, Thoracic, and Mediastinal Disorders: epistaxis (9%)
- Nervous System Disorders: dizziness (9%) [including dizziness, postural dizziness, and vertigo] and dysgeusia (6%)
- Skin and Subcutaneous Disorders: pruritus (5%) and skin hyperpigmentation (4.3%) [including skin hyperpigmentation, skin discoloration, pigmentation disorder]
- Eye Disorders: blurred vision (4%) [including blurred vision, visual impairment]
- Metabolism and Nutrition Disorders: dehydration (3.2%)
- Gastrointestinal Disorders: abdominal distension (2.6%), flatulence (1.7%) and gastritis (0.9%)
- Blood and Lymphatic System Disorders: febrile neutropenia (1.7%)
- Injury, Poisoning, and Procedural Complications: infusion-related reactions (1.4%) [including administration related reaction, anaphylactic reaction, hypersensitivity, infusion-related reaction and infusion-related hypersensitivity reaction]
Table 18: Selected Laboratory Abnormalities in HER2-positive (IHC 3+) Patients Treated with ENHERTU in DESTINY-Breast01, DESTINY-PanTumor02, DESTINY-Lung01, and DESTINY-CRC02 Laboratory Parameter ENHERTU 5.4 mg/kg
N= 347*All Grades
%Grades 3 or 4
%- *
- Percentages were calculated using the number of patients with worsening laboratory values from baseline and the number of patients with both baseline and post-treatment measurements as the denominator.
Hematology Decreased white blood cell count 75 11 Decreased hemoglobin 67 10 Decreased neutrophil count 66 21 Decreased lymphocyte count 58 21 Decreased platelet count 51 7 Chemistry Increased aspartate aminotransferase 45 1.5 Increased alanine aminotransferase 44 1.5 Increased blood alkaline phosphatase 36 1.2 Decreased blood potassium 29 6 Decreased sodium 22 2.9 Increased blood bilirubin 15 0.6 Increased blood creatinine 14 0.6 -
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on its mechanism of action, ENHERTU can cause fetal harm when administered to a pregnant woman. There are no available data on the use of ENHERTU in ...
8.1 Pregnancy
Risk Summary
Based on its mechanism of action, ENHERTU can cause fetal harm when administered to a pregnant woman. There are no available data on the use of ENHERTU in pregnant women. In postmarketing reports, use of a HER2-directed antibody during pregnancy resulted in cases of oligohydramnios manifesting as fatal pulmonary hypoplasia, skeletal abnormalities, and neonatal death (see Data). Based on its mechanism of action, the topoisomerase inhibitor component of ENHERTU, DXd, can also cause embryo-fetal harm when administered to a pregnant woman because it is genotoxic and targets actively dividing cells [see Clinical Pharmacology (12.1), Nonclinical Toxicology (13.1)]. Advise patients of the potential risks to a fetus.
There are clinical considerations if ENHERTU is used in pregnant women, or if a patient becomes pregnant within 7 months after the last dose of ENHERTU (see Clinical Considerations).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Human Data
There are no available data on the use of ENHERTU in pregnant women. In postmarketing reports in pregnant women receiving a HER2-directed antibody, cases of oligohydramnios manifesting as fatal pulmonary hypoplasia, skeletal abnormalities, and neonatal death have been reported. These case reports described oligohydramnios in pregnant women who received a HER2-directed antibody either alone or in combination with chemotherapy. In some case reports, amniotic fluid index increased after use of a HER2-directed antibody was stopped.
8.2 Lactation
Risk Summary
There is no data regarding the presence of fam-trastuzumab deruxtecan-nxki in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in a breastfed child, advise women not to breastfeed during treatment with ENHERTU and for 7 months after the last dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify pregnancy status of females of reproductive potential prior to initiation of ENHERTU.
Contraception
Females
ENHERTU can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with ENHERTU and for 7 months after the last dose.
Males
Because of the potential for genotoxicity, advise male patients with female partners of reproductive potential to use effective contraception during treatment with ENHERTU and for 4 months after the last dose [see Nonclinical Toxicology (13.1)].
Infertility
Based on findings in animal toxicity studies, ENHERTU may impair male reproductive function and fertility [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness of ENHERTU have not been established in pediatric patients.
Animal Data
Juvenile animal studies have not been conducted with fam-trastuzumab deruxtecan-nxki. In a six-week repeat-dose toxicity study in rats, intravenous administration of fam-trastuzumab deruxtecan-nxki resulted in incisor tooth toxicity including single cell necrosis in the base area (e.g., ameloblasts, odontoblasts) and degeneration of the enamel at ≥60 mg/kg (approximately ≥9 times the human recommended dose of 5.4 mg/kg based on AUC), abnormal formation or hypoplasia of the dentin, hemorrhage in the sub-enamel organ tissue, and focal lack of the cementum at 197 mg/kg (approximately 19 times the human recommended dose of 5.4 mg/kg based on AUC). Degeneration of the enamel organ, abnormal dentin formation, hemorrhage in the sub-enamel organ tissue, focal lack of the cementum, and root fracture were observed at 197 mg/kg following a 9-week recovery period.
8.5 Geriatric Use
Of the 1741 patients with HER2-positive, HER2-low, or HER2-ultralow breast cancer treated with ENHERTU 5.4 mg/kg, 24% were 65 years or older and 4.9% were 75 years or older. No overall differences in efficacy within clinical studies were observed between patients ≥65 years of age compared to younger patients. There was a higher incidence of Grade 3-4 adverse reactions observed in patients aged 65 years or older (61%) as compared to younger patients (52%).
Of the 101 patients with HER2-mutant unresectable or metastatic NSCLC treated with ENHERTU 5.4 mg/kg, 40% were 65 years or older and 8% were 75 years or older. No overall differences in efficacy or safety were observed between patients ≥65 years of age compared to younger patients.
Of the 125 patients with HER2-positive locally advanced or metastatic gastric or GEJ adenocarcinoma treated with ENHERTU 6.4 mg/kg in DESTINY-Gastric01, 56% were 65 years or older and 14% were 75 years or older. No overall differences in efficacy or safety were observed between patients ≥65 years of age compared to younger patients.
Of the 192 patients with HER2-positive (IHC 3+) unresectable or metastatic solid tumors treated with ENHERTU 5.4 mg/kg in DESTINY-PanTumor02, DESTINY-Lung01 or DESTINY-CRC02, 39% were 65 years or older and 9% were 75 years or older. No overall differences in efficacy or safety were observed between patients ≥65 years of age compared to younger patients.
8.6 Renal Impairment
No dose adjustment of ENHERTU is required in patients with mild (creatinine clearance [CLcr] ≥60 and <90 mL/min) or moderate (CLcr ≥30 and <60 mL/min) renal impairment [see Clinical Pharmacology (12.3)]. A higher incidence of Grade 1 and 2 ILD/pneumonitis has been observed in patients with moderate renal impairment [see Warnings and Precautions (5.1)]. Monitor patients with moderate renal impairment more frequently. The recommended dosage of ENHERTU has not been established for patients with severe renal impairment (CLcr <30 mL/min) [see Clinical Pharmacology (12.3)].
Close8.7 Hepatic Impairment
No dose adjustment of ENHERTU is required in patients with mild (total bilirubin ≤ULN and any AST >ULN or total bilirubin >1 to 1.5 times ULN and any AST) or moderate (total bilirubin >1.5 to 3 times ULN and any AST) hepatic impairment. In patients with moderate hepatic impairment, due to potentially increased exposure, closely monitor for increased toxicities related to the topoisomerase inhibitor, DXd [see Dosage and Administration (2.3)]. The recommended dosage of ENHERTU has not been established for patients with severe hepatic impairment (total bilirubin >3 times ULN and any AST) [see Clinical Pharmacology (12.3)].
-
11 DESCRIPTIONFam-trastuzumab deruxtecan-nxki is a HER2-directed antibody and topoisomerase inhibitor conjugate. Fam-trastuzumab deruxtecan-nxki is an antibody-drug conjugate (ADC) composed of three components ...
Fam-trastuzumab deruxtecan-nxki is a HER2-directed antibody and topoisomerase inhibitor conjugate. Fam-trastuzumab deruxtecan-nxki is an antibody-drug conjugate (ADC) composed of three components: 1) a humanized anti-HER2 IgG1 monoclonal antibody (mAb), covalently linked to 2) a topoisomerase inhibitor, via 3) a tetrapeptide-based cleavable linker. Deruxtecan is composed of a protease-cleavable maleimide tetrapeptide linker and the topoisomerase inhibitor, DXd, which is an exatecan derivative.
The antibody is produced in Chinese hamster ovary cells by recombinant DNA technology, and the topoisomerase inhibitor and linker are produced by chemical synthesis. Approximately 8 molecules of deruxtecan are attached to each antibody molecule. Fam-trastuzumab deruxtecan-nxki has the following structure:
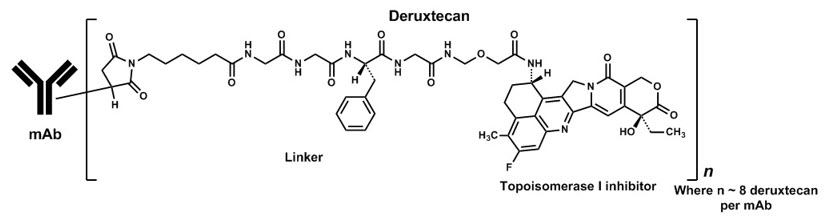
ENHERTU (fam-trastuzumab deruxtecan-nxki) is a sterile, white to yellowish white, preservative-free lyophilized powder in single-dose vials. Each vial delivers 100 mg of fam-trastuzumab deruxtecan-nxki, L-histidine (4.45 mg), L-histidine hydrochloride monohydrate (20.2 mg), polysorbate 80 (1.5 mg), and sucrose (450 mg). Following reconstitution with 5 mL of Sterile Water for Injection, USP, the resulting concentration of fam-trastuzumab deruxtecan-nxki is 20 mg/mL with a pH of 5.5. The resulting solution is administered by intravenous infusion following dilution.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Fam-trastuzumab deruxtecan-nxki is a HER2-directed antibody-drug conjugate. The antibody is a humanized anti-HER2 IgG1. The small molecule, DXd, is a topoisomerase I ...
12.1 Mechanism of Action
Fam-trastuzumab deruxtecan-nxki is a HER2-directed antibody-drug conjugate. The antibody is a humanized anti-HER2 IgG1. The small molecule, DXd, is a topoisomerase I inhibitor attached to the antibody by a cleavable linker. Following binding to HER2 on tumor cells, fam-trastuzumab deruxtecan-nxki undergoes internalization and intracellular linker cleavage by lysosomal enzymes. Upon release, the membrane-permeable DXd causes DNA damage and apoptotic cell death.
12.2 Pharmacodynamics
12.3 Pharmacokinetics
The pharmacokinetics of fam-trastuzumab deruxtecan-nxki was evaluated in patients with cancer. Following a single dose, exposures (Cmax and AUC) of fam-trastuzumab deruxtecan-nxki and released topoisomerase inhibitor (DXd) increased proportionally over a dose range of 3.2 mg/kg to 8 mg/kg (approximately 0.6 to 1.5 times the recommended dose in breast cancer, NSCLC, and HER2-positive (IHC 3+) solid tumors and 0.5 to 1.25 times the recommended dose in gastric cancer).
At the recommended dosage of ENHERTU for patients with metastatic breast cancer, NSCLC, and HER2-positive (IHC 3+) solid tumors, the geometric mean (coefficient of variation [CV]%) Cmax of fam-trastuzumab deruxtecan-nxki and DXd were 132 µg/mL (20%) and 4.7 ng/mL (48%), respectively, and the AUC of fam-trastuzumab deruxtecan-nxki and DXd were 772 µg∙day/mL (27%) and 29 ng∙day/mL (48%), respectively. Accumulation of fam-trastuzumab deruxtecan-nxki was approximately 35% at steady-state (Cycle 3).
At the recommended dosage of ENHERTU for patients with HER2-positive gastric cancer, the geometric mean Cmax,ss of fam-trastuzumab deruxtecan-nxki and DXd were 126 µg/mL (18%) and 5.2 ng/mL (42%), respectively, and the AUCss of fam-trastuzumab deruxtecan-nxki and DXd were 743 µg∙day/mL (26%) and 33 ng∙day/mL (43%), respectively. Accumulation of fam-trastuzumab deruxtecan-nxki was approximately 39% at steady-state (Cycle 3).
Distribution
The estimated volume of distribution of the central compartment (Vc) of fam-trastuzumab deruxtecan-nxki was 2.68 L.
DXd plasma protein binding is approximately 97% and the blood-to-plasma ratio is approximately 0.6, in vitro.
Elimination
The median elimination half-life (t1/2) of fam-trastuzumab deruxtecan-nxki is 5.4-5.7 days. The estimated systemic clearance of fam-trastuzumab deruxtecan-nxki was 0.41 L/day.
The median elimination half-life (t1/2) of DXd is 5.4-6.1 days. The estimated systemic clearance of DXd was 18.3 L/h.
Specific Populations
No clinically significant differences in the pharmacokinetics of fam-trastuzumab deruxtecan-nxki or DXd were observed for age (20-96 years); race (Asian vs Non-Asian), including White, and Black or African American; sex; body weight (27.3-125.4 kg); tumor types; mild hepatic impairment; mild or moderate renal impairment.
The pharmacokinetics of fam-trastuzumab deruxtecan-nxki or DXd in patients with moderate to severe hepatic impairment or severe renal impairment is unknown.
Drug Interaction Studies
Clinical Studies
In Vitro Studies
Effects of DXd on CYP Enzymes: DXd does not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A nor induce CYP1A2, CYP2B6, or CYP3A.
Close12.6 Immunogenicity
The observed incidence of anti-drug antibodies (ADA) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of ADA in the studies described below with the incidence of ADA in other studies, including those of fam-trastuzumab deruxtecan-nxki or of other fam-trastuzumab deruxtecan products.
Among patients who received ENHERTU as a single agent and were tested for ADA over a 6 to 9 month treatment period in 13 clinical trials, anti-fam-trastuzumab deruxtecan-nxki antibodies developed in 2.2% (49/2,231) of patients who received ENHERTU 5.4 mg/kg every three weeks and in 2.6% (21/793) of patients who received ENHERTU 6.4 mg/kg every three weeks. There was no identified clinically significant effect of ADAs on pharmacokinetics or safety of fam-trastuzumab deruxtecan-nxki. Because of the low occurrence of ADA, the effect of ADAs on the effectiveness of fam-trastuzumab deruxtecan-nxki is unknown.
Neutralizing antibodies against fam-trastuzumab deruxtecan-nxki were detected in 6% (4/70) of patients who were ADA-positive. Due to the limited number of patients who developed neutralizing antibodies against fam-trastuzumab deruxtecan-nxki, the effect of neutralizing antibodies is unknown.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies have not been conducted with fam-trastuzumab deruxtecan-nxki. The topoisomerase inhibitor component of ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with fam-trastuzumab deruxtecan-nxki.
The topoisomerase inhibitor component of fam-trastuzumab deruxtecan-nxki, DXd, was clastogenic in both an in vivo rat bone marrow micronucleus assay and an in vitro Chinese hamster lung chromosome aberration assay and was not mutagenic in an in vitro bacterial reverse mutation assay.
Fertility studies have not been conducted with fam-trastuzumab deruxtecan-nxki. In a six-week repeat-dose toxicity study in rats, intravenous administration of fam-trastuzumab deruxtecan-nxki resulted in spermatid retention at 20 mg/kg and 60 mg/kg (approximately 4 and 9 times the human recommended dose of 5.4 mg/kg based on AUC, respectively). Decreased testes and epididymides weights, tubular atrophy/degeneration in testes, and reduced sperm count in epididymides were observed at a dose of 197 mg/kg (19 times the human recommended dose of 5.4 mg/kg based on AUC). In a three-month repeat-dose toxicity study in monkeys, intravenous administration of fam-trastuzumab deruxtecan-nxki resulted in decreased numbers of round spermatids in the testes at seminiferous tubule stages V to VI at ≥30 mg/kg (≥7 times the human recommended dose of 5.4 mg/kg based on AUC). Evidence of reversibility was observed in monkeys by the end of a three-month recovery period.
-
14 CLINICAL STUDIES14.1 HER2-Positive Metastatic Breast Cancer - DESTINY-Breast03 - The efficacy of ENHERTU was evaluated in study DESTINY-Breast03 (NCT03529110), a multicenter, open-label, randomized trial ...
14.1 HER2-Positive Metastatic Breast Cancer
DESTINY-Breast03
The efficacy of ENHERTU was evaluated in study DESTINY-Breast03 (NCT03529110), a multicenter, open-label, randomized trial that enrolled 524 patients with HER2-positive, unresectable and/or metastatic breast cancer who received prior trastuzumab and taxane therapy for metastatic disease or developed disease recurrence during or within 6 months of completing adjuvant therapy. HER2 expression was based on archival tissue tested at a central laboratory prior to enrollment with HER2 positivity defined as HER2 IHC 3+ or ISH positive. Patients were excluded for a history of ILD/pneumonitis requiring treatment with steroids, ILD/pneumonitis at screening, or clinically significant cardiac disease. Patients were also excluded for untreated and symptomatic brain metastases, ECOG performance status >1, or prior treatment with an anti-HER2 antibody-drug conjugate in the metastatic setting.
Patients were randomized 1:1 to receive either ENHERTU 5.4 mg/kg (N=261) or ado-trastuzumab emtansine 3.6 mg/kg (N=263) by intravenous infusion every 3 weeks until unacceptable toxicity or disease progression. Randomization was stratified by hormone receptor status, prior treatment with pertuzumab, and visceral versus non-visceral disease. Tumor imaging was obtained every 6 weeks and CT/MRI of the brain was mandatory for all patients at baseline. The major efficacy outcomes were progression-free survival (PFS) as assessed by blinded independent central review (BICR) based on Response Evaluation Criteria in Solid Tumors (RECIST) v.1.1 and overall survival (OS). Confirmed objective response rate (ORR) was an additional outcome measure.
The median age was 54 years (range: 20-83); 80% were <65 years; 99.6% were female; 60% were Asian, 27% were White, and 3.6% were Black; 11% of patients were of Hispanic/Latino ethnicity. Patients had an ECOG performance status of 0 (63%) or 1 (37%) at baseline. Seventy-three percent had visceral disease, 16% had brain metastases at baseline, 52% were hormone receptor positive (HR+), and 48% of patients had received one line of prior systemic therapy in the metastatic setting. The percentage of patients who had not received prior treatment for metastatic disease was 10%.
Efficacy results are summarized in Table 19 and Figures 1 and 2.
Table 19: Efficacy Results in DESTINY-Breast03 Efficacy Parameter ENHERTU
5.4 mg/kgAdo-trastuzumab emtansine
3.6 mg/kgCI = confidence interval; NR = not reached; NE = not estimable - *
- The stratified log-rank test p-value is compared with the allocated alpha of 0.0002 for this interim analysis (with 73% of the planned number of events for final analysis).
- †
- The stratified log-rank test p-value is compared with the allocated alpha of 0.013 for this interim analysis (with 68% of the planned number of events for final analysis).
- ‡
- Analysis was performed based on the patients with measurable disease assessed by BICR at baseline.
Progression-Free Survival (PFS) per BICR N 261 263 Number of events (%) 87 (33.3) 158 (60.1) Median, months (95% CI) NR (18.5, NE) 6.8 (5.6, 8.2) Hazard ratio (95% CI) 0.28 (0.22, 0.37) p-value* p< 0.0001 Overall Survival (OS) N 261 263 Number of events (%) 72 (27.6) 97 (36.9) Median, months (95% CI) NR (40.5, NE) NR (34.0, NE) Hazard ratio (95% CI) 0.64 (0.47, 0.87) p-value† p=0.0037 Confirmed Objective Response Rate (ORR) per BICR‡ N 248 241 n (%) 205 (82.7) 87 (36.1) 95% CI (77.4, 87.2) (30.0, 42.5) Complete Response n (%) 39 (15.7) 20 (8.3) Partial Response n (%) 166 (66.9) 67 (27.8) Figure 1: Kaplan-Meier Plot of Progression-Free Survival per BICR (Intent-to-Treat Analysis Set) 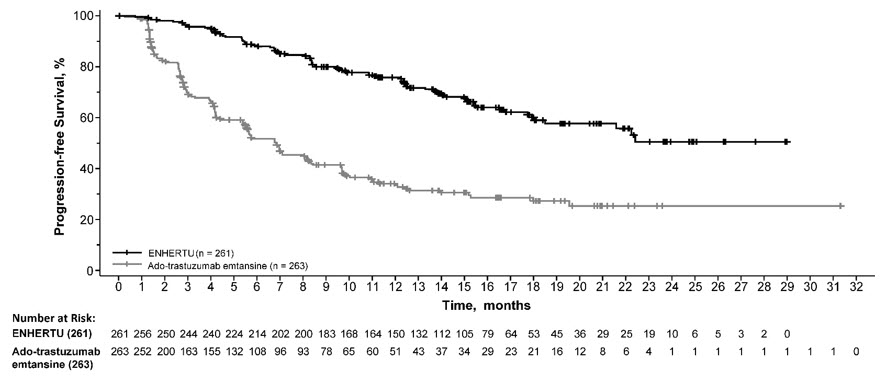
Figure 2: Kaplan-Meier Plot of Overall Survival 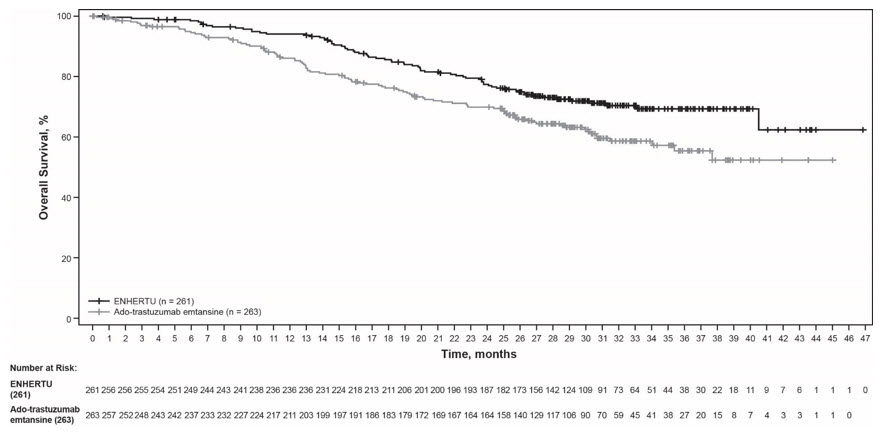
DESTINY-Breast02
The efficacy of ENHERTU was evaluated in study DESTINY-Breast02 (NCT03523585), a multicenter, open-label, randomized study that enrolled 608 patients with HER2-positive, unresectable and/or metastatic breast cancer who were previously treated with ado-trastuzumab emtansine. HER2 expression was based on archival tissue tested at a central laboratory prior to enrollment with HER2 positivity defined as HER2 IHC 3+ or ISH positive. Patients were randomized 2:1 to receive either ENHERTU 5.4 mg/kg (N=406) by intravenous infusion every 3 weeks or treatment of physician's choice (TPC) (N=202, trastuzumab plus capecitabine or lapatinib plus capecitabine) until unacceptable toxicity or disease progression. Randomization was stratified by hormone receptor status, prior treatment with pertuzumab, and visceral versus non-visceral disease. The major efficacy outcomes were PFS as assessed by BICR based on RECIST v1.1 and OS.
The median age was 54 years (range: 22 to 88); 80% were <65 years; 99% were female; 63% were White, 29% were Asian, and 3% were Black or African American; 11% of patients were of Hispanic/Latino ethnicity. Patients had an ECOG performance status of 0 (57%) or 1 (42%) at baseline. Seventy-eight percent had visceral disease, 18% had brain metastases at baseline, 59% were hormone receptor positive (HR+), and 5% of patients had received one line of prior systemic therapy in the metastatic setting.
Efficacy results are summarized in Table 20 and Figures 3 and 4.
Table 20: Efficacy Results in DESTINY-Breast02 Efficacy Parameter ENHERTU
N=406Treatment of Physician's Choice
N=202CI = confidence interval; NE=not estimable - *
- The stratified log-rank test p-value is compared with the allocated alpha of 0.004 for this interim analysis (with 53% of the planned number of events for final analysis)
PFS per BICR Number of events (%) 200 (49.3) 125 (61.9) Median, months (95% CI) 17.8 (14.3, 20.8) 6.9 (5.5, 8.4) Hazard ratio (95% CI) 0.36 (0.28, 0.45) p-value p<0.0001 Overall Survival (OS) Number of events (%) 143 (35.2) 86 (42.6) Median, months (95% CI) 39.2 (32.7, NE) 26.5 (21.0, NE) Hazard ratio (95% CI) 0.66 (0.50, 0.86) p-value* p=0.0021 Confirmed Objective Response Rate (ORR) per BICR n (%) 283 (69.7) 59 (29.2) 95% CI (65.0, 74.1) (23.0, 36.0) Complete Response n (%) 57 (14.0) 10 (5.0) Partial Response n (%) 226 (55.7) 49 (24.3) Duration of Response per BICR Median, months (95% CI) 19.6 (15.9, NE) 8.3 (5.8, 9.5) Figure 3: Kaplan-Meier Plot of Progression-free Survival Per BICR 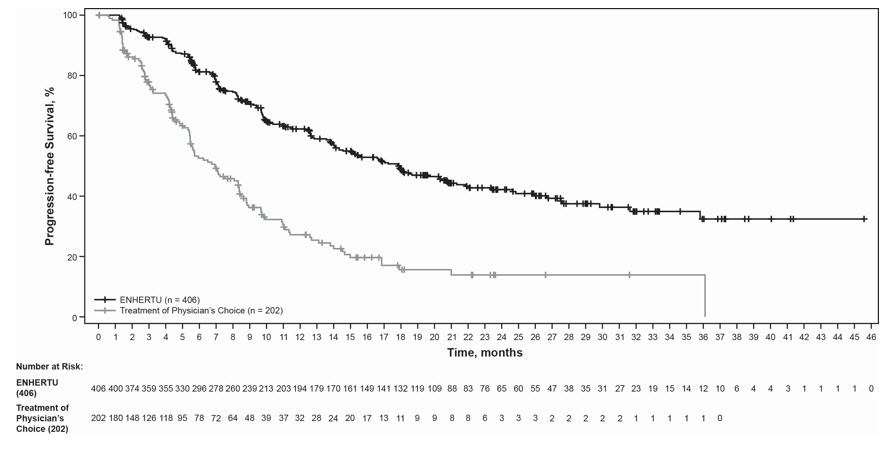
Figure 4: Kaplan-Meier Plot of Overall Survival 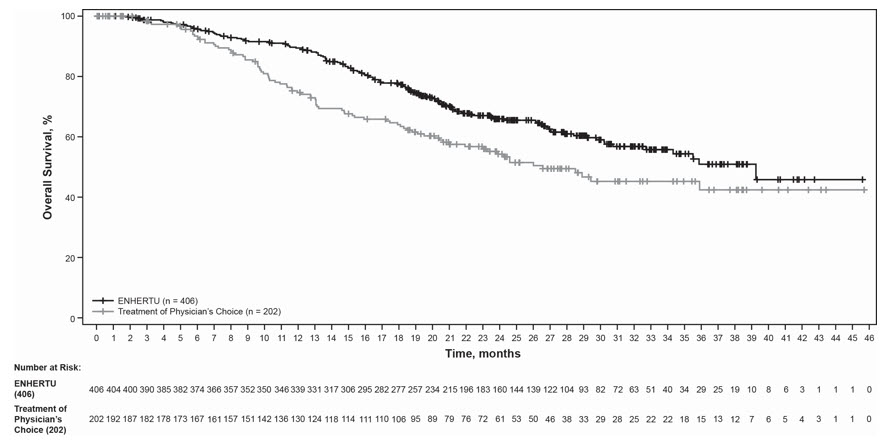
DESTINY-Breast01
The efficacy of ENHERTU was evaluated in study DESTINY-Breast01 (NCT03248492), a multicenter, single-arm, trial that enrolled 184 female patients with HER2-positive, unresectable and/or metastatic breast cancer who had received two or more prior anti-HER2 therapies. Patients were excluded for a history of treated ILD or current ILD at screening. Patients were also excluded for history of clinically significant cardiac disease, active brain metastases, and ECOG performance status >1. HER2 expression was based on archival tissue tested at a central laboratory prior to enrollment with HER2 positivity defined as HER2 IHC 3+ or ISH positive.
Patients received ENHERTU 5.4 mg/kg by intravenous infusion every 3 weeks until unacceptable toxicity or disease progression. Tumor imaging was obtained every 6 weeks and CT/MRI of the brain was mandatory for patients with brain metastases at baseline. The major efficacy outcomes were confirmed objective response rate (ORR) assessed by independent central review (ICR) using RECIST v1.1 and duration of response (DOR).
The median age was 55 years (range: 28-96); 76% of patients were <65 years. All 184 patients were female, and the majority were White (55%) or Asian (38%). Patients had an ECOG performance status of 0 (55%) or 1 (44%) at baseline. Ninety-two percent had visceral disease, 29% had bone metastases, and 13% had brain metastases. Fifty-three percent were HR+. Sum of diameters of target lesions were <5 cm in 42%, and ≥5 cm in 50% (not evaluable by central review in 8% of patients).
The median number of prior cancer regimens in the locally advanced/metastatic setting was 5 (range: 2-17). All patients received prior trastuzumab, ado-trastuzumab emtansine, and 66% had prior pertuzumab.
Efficacy results are summarized in Table 21.
Table 21: Efficacy Results by Independent Central Review in DESTINY-Breast01 Efficacy Parameter DESTINY-Breast01
N=184ORR 95% CI calculated using Clopper-Pearson method. Confirmed Objective Response Rate (95% CI) 60.3% (52.9, 67.4) Complete Response 4.3% Partial Response 56.0% Duration of Response*
Median, months (95% CI)†14.8 (13.8, 16.9) 14.2 HER2-Low and HER2-Ultralow Metastatic Breast Cancer
DESTINY-Breast06
The efficacy of ENHERTU was evaluated in study DESTINY-Breast06 (NCT04494425), a randomized (1:1), multicenter, open-label study that randomized 866 adult patients with advanced or metastatic HR+ breast cancer with HER2-low (IHC 1+ or IHC 2+/ISH-) or HER2-ultralow (IHC 0 with membrane staining) expression as determined by Ventana's PATHWAY anti-HER-2/neu (4B5) Rabbit Monoclonal Primary Antibody assay and evaluated at a central laboratory. HER2-ultralow is defined as membrane staining that is seen in >0 and ≤10% of tumor cells. Patients were eligible if they had disease progression on (a) at least 2 lines of endocrine therapy in the metastatic setting or (b) one line of endocrine therapy in the metastatic setting and demonstrated progression within 24 months of the start of adjuvant endocrine therapy, or within 6 months of starting first line endocrine therapy in combination with a CDK 4/6 inhibitor in the metastatic setting. Patients with prior chemotherapy in the neoadjuvant or adjuvant setting were eligible if they had a disease-free interval greater than 12 months. The study excluded patients with prior chemotherapy for advanced or metastatic disease, patients with a history of ILD/pneumonitis requiring treatment with steroids or ILD/pneumonitis at screening, uncontrolled or significant cardiovascular disease, untreated and symptomatic brain metastases, or ECOG performance status >1.
Patients were randomized to receive either ENHERTU 5.4 mg/kg (N=436) by intravenous infusion every three weeks or physician's choice of single agent chemotherapy (N=430, capecitabine 60%, nab-paclitaxel 24%, or paclitaxel 16%). Randomization was stratified by prior CDK4/6 inhibitor use (yes or no), prior taxane use in the non-metastatic setting (yes or no), and HER2 IHC status of tumor samples (IHC 2+/ISH- vs IHC 1+ vs IHC 0 with membrane staining). Treatment with ENHERTU was administered until disease progression, death, withdrawal of consent, or unacceptable toxicity.
The major efficacy outcome measure was PFS in patients with HER2-low breast cancer assessed by BICR based on RECIST v1.1. Additional efficacy outcome measures were PFS assessed by BICR based on RECIST v1.1 in the overall population, OS in HER2-low patients, and OS in the overall population.
The median age was 57 years (range: 28 to 87); 31% were age 65 or older; 99.9% were female; 53% were White, 35% were Asian, 0.8% were Black or African American, 0.1% were American Indian or Alaska Native, and 7% were of Hispanic/Latino ethnicity. Patients had an ECOG performance status of 0 (59%) or 1 (39%) at baseline; 18% were IHC 0 with membrane staining, 55% were IHC 1+, 27% were IHC 2+/ISH-; 67% had liver metastases, 32% had lung metastases, 8% had brain metastases, and 3% had bone-only metastases. Patients had a median of 2 prior lines of endocrine therapy in the metastatic setting (range: 1 to 5) with 17% having 1 and 68% having 2. Eighty-nine percent of patients had prior endocrine therapy in combination with CDK4/6i treatment in the metastatic setting, 41% had prior taxane use in the non-metastatic setting.
Efficacy results are summarized in Table 22 and Figure 5. At the time of the PFS final analysis, overall survival (OS) data was immature, and a total of 335 (39%) of patients had died across both study arms in the overall population.
Table 22: Efficacy Results in DESTINY-Breast06 Efficacy Parameter HER2-low Overall Population
(HER2-low and HER2-ultralow)ENHERTU
(N=359)Chemotherapy
(N=354)ENHERTU
(N=436)Chemotherapy
(N=430)CI = confidence interval Progression Free Survival (PFS) per BICR Number of events (%) 225 (62.7) 232 (65.5) 269 (61.7) 271 (63.0) Median, months (95% CI) 13.2 (11.4, 15.2) 8.1 (7.0, 9.0) 13.2 (12.0, 15.2) 8.1 (7.0, 9.0) Hazard ratio (95% CI) 0.62 (0.52, 0.75)* 0.64 (0.54, 0.76)† p-value <0.0001* <0.0001† Confirmed Objective Response Rate (ORR) per BICR‡ N 326 324 393 389 n (%) 202 (62.0) 114 (35.2) 246 (62.6) 134 (34.4) 95% CI 56.5, 67.3 30.0, 40.7 57.6, 67.4 29.7, 39.4 Complete Response n (%) 9 (2.8) 0 10 (2.5) 0 Partial Response n (%) 193 (59.2) 114 (35.2) 236 (60.1) 134 (34.4) Duration of Response (DOR) per BICR‡ Median, months (95% CI) 14.1 (11.9, 15.9) 8.6 (6.7, 11.3) 14.3 (12.5, 15.9) 8.6 (6.9, 11.5) In an exploratory analysis of the HER2-ultralow subgroup (n=153), median PFS was 15.1 months (95% CI: 10.0, 17.3) in patients randomized to ENHERTU and 8.3 months (95% CI: 5.8, 15.2) in patients randomized to chemotherapy with a hazard ratio of 0.76 (95% CI: 0.49, 1.17). Confirmed ORR (BICR) was 65.7% (95% CI: 53.1, 76.8) and 30.8% (95% CI: 19.9, 43.4) in patients randomized to ENHERTU and chemotherapy and with measurable disease at baseline, respectively. Median DOR was 14.3 months (95% CI: 11.8, not estimable) and 14.1 months (95% CI: 5.9, not estimable) in patients randomized to ENHERTU and chemotherapy, respectively.
Figure 5: Kaplan-Meier Plot of Progression Free Survival (Overall Population) 
DESTINY-Breast04
The efficacy of ENHERTU was evaluated in study DESTINY-Breast04 (NCT03734029), a randomized, multicenter, open-label study that enrolled 557 adult patients with unresectable or metastatic HER2-low breast cancer. The study included 2 cohorts: 494 hormone receptor-positive (HR+) patients and 63 hormone receptor-negative (HR-) patients. HER2-low expression was defined as IHC 1+ or IHC 2+/ISH-, as determined at a central laboratory using Ventana's PATHWAY anti-HER-2/neu (4B5) Rabbit Monoclonal Primary Antibody assay. Patients must have received chemotherapy in the metastatic setting or have developed disease recurrence during or within 6 months of completing adjuvant chemotherapy. Patients who were HR+ must have received at least one endocrine therapy or be ineligible for endocrine therapy. Patients were randomized 2:1 to receive either ENHERTU 5.4 mg/kg (N=373) by intravenous infusion every 3 weeks or physician's choice of chemotherapy (N=184, eribulin, capecitabine, gemcitabine, nab paclitaxel, or paclitaxel). Randomization was stratified by HER2 IHC status of tumor samples (IHC 1+ or IHC 2+/ISH-), number of prior lines of chemotherapy in the metastatic setting (1 or 2), and HR status/prior CDK4/6i treatment (HR+ with prior CDK4/6 inhibitor treatment, HR+ without prior CDK4/6 inhibitor treatment, or HR-). Treatment was administered until disease progression, death, withdrawal of consent, or unacceptable toxicity. The study excluded patients with a history of ILD/pneumonitis requiring treatment with steroids or ILD/pneumonitis at screening and clinically significant cardiac disease. Patients were also excluded for untreated or symptomatic brain metastases or ECOG performance status >1.
The major efficacy outcome measure was PFS in patients with HR+ breast cancer assessed by BICR based on RECIST v1.1. Additional efficacy outcome measures were PFS assessed by BICR based on RECIST v1.1 in the overall population (all randomized HR+ and HR- patients), OS in HR+ patients, and OS in the overall population.
The median age was 57 years (range: 28 to 81); 24% were age 65 or older; 99.6% were female; 48% were White, 40% were Asian, and 2% were Black or African American; 3.8% of patients were of Hispanic/Latino ethnicity. Patients had an ECOG performance status of 0 (55%) or 1 (45%) at baseline; 58% were IHC 1+, 42% were IHC 2+/ISH-; 70% had liver metastases, 33% had lung metastases, and 6% had brain metastases. In the metastatic setting, patients had a median of 3 prior lines of systemic therapy (range: 1 to 9) with 58% having 1 and 41% having 2 prior chemotherapy regimens; 3.9% were early progressors (progression in the neo/adjuvant setting). In HR+ patients, the median number of prior lines of endocrine therapy was 2 (range: 0 to 9) and 70% had prior CDK4/6i treatment.
Efficacy results are summarized in Table 23 and Figures 6 and 7.
Table 23: Efficacy Results in DESTINY-Breast04 Efficacy Parameter HR+ Cohort Overall Population
(HR+ and HR- Cohorts)ENHERTU
(N=331)Chemotherapy
(N=163)ENHERTU
(N=373)Chemotherapy
(N=184)CI = confidence interval Overall Survival Number of events (%) 126 (38.1) 73 (44.8) 149 (39.9) 90 (48.9) Median, months (95% CI) 23.9 (20.8, 24.8) 17.5 (15.2, 22.4) 23.4 (20.0, 24.8) 16.8 (14.5, 20.0) Hazard ratio (95% CI) 0.64 (0.48, 0.86) 0.64 (0.49, 0.84) p-value 0.0028 0.001 Progression-Free Survival per BICR Number of events (%) 211 (63.7) 110 (67.5) 243 (65.1) 127 (69.0) Median, months (95% CI) 10.1 (9.5, 11.5) 5.4 (4.4, 7.1) 9.9 (9.0, 11.3) 5.1 (4.2, 6.8) Hazard ratio (95% CI) 0.51 (0.40, 0.64) 0.50 (0.40, 0.63) p-value <0.0001 <0.0001 Confirmed Objective Response Rate per BICR n (%) 175 (52.9) 27 (16.6) 195 (52.3) 30 (16.3) 95% CI 47.3, 58.4 11.2, 23.2 47.1, 57.4 11.3, 22.5 Complete Response n (%) 12 (3.6) 1 (0.6) 13 (3.5) 2 (1.1) Partial Response n (%) 164 (49.5) 26 (16.0) 183 (49.1) 28 (15.2) Duration of Response per BICR Median, months (95% CI) 10.7 (8.5, 13.7) 6.8 (6.5, 9.9) 10.7 (8.5, 13.2) 6.8 (6.0, 9.9) Figure 6: Kaplan-Meier Plot of Overall Survival (Overall Population) 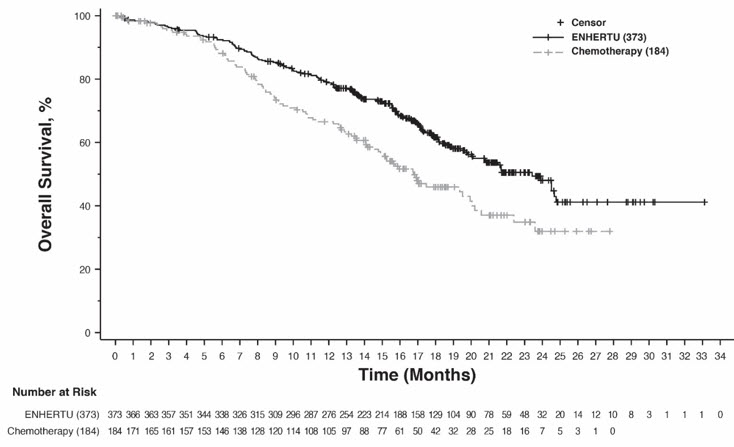
Figure 7: Kaplan-Meier Plot of Progression-Free Survival (Overall Population) 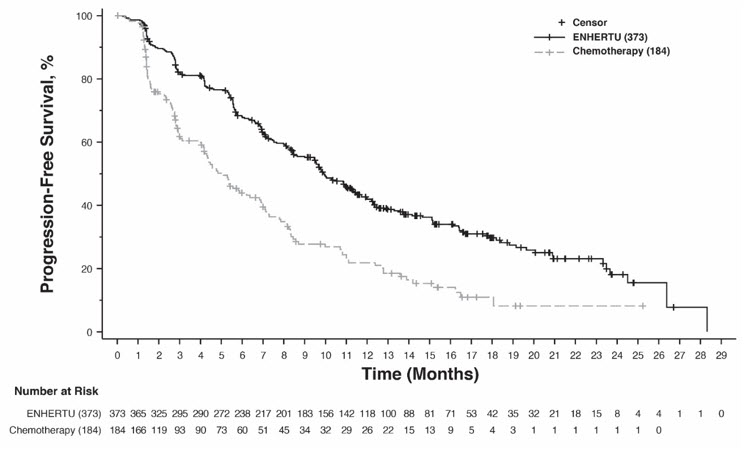
14.3 HER2-Mutant Unresectable or Metastatic Non-Small Cell Lung Cancer
ENHERTU was evaluated in DESTINY-Lung01 (NCT03505710) and at two dose levels in DESTINY-Lung02 (NCT04644237). Patients were prospectively selected for treatment with ENHERTU based on the presence of activating HER2 (ERBB2) mutations by local testing using tissue. Samples from DESTINY-Lung01 were retrospectively tested using Oncomine™ Dx Target Test (Life Technologies Corporation, Tissue-test) and Guardant360® CDx test (Guardant Health Inc., Plasma test). Demographic and baseline disease characteristics were similar for patients in DESTINY-Lung01 and DESTINY-Lung02, except for race (34% Asian vs 79% Asian, respectively). Response rates were consistent across dose levels. Increased rates of ILD/pneumonitis were observed at the higher dose. The approved recommended dose of 5.4 mg/kg intravenously every 3 weeks in the DESTINY-Lung02 study is described below [see Adverse Reactions (6.1)].
The efficacy of ENHERTU was evaluated in DESTINY-Lung02, a multicenter, multicohort, randomized, blinded, dose-optimization trial. Eligible patients were required to have unresectable or metastatic HER2-mutant non-squamous NSCLC with disease progression after one prior systemic therapy. Patients with a history of steroid dependent ILD/pneumonitis, clinically significant cardiac disease, clinically active brain metastases, and ECOG performance status >1 were excluded. Patients received ENHERTU 5.4 mg/kg by intravenous infusion every 3 weeks until disease progression or unacceptable toxicity. Tumor imaging was obtained every 6 weeks and CT/MRI of the brain was mandatory for patients with stable brain metastases at baseline.
Results from an interim efficacy analysis in a pre-specified patient cohort are described below. The major efficacy outcomes were confirmed ORR as assessed by BICR using RECIST v1.1 and DOR.
The median age was 58 years (range 30 to 78); 69% were female; 79% were Asian, 12% were White, and 10% were other races; 29% had an ECOG performance status of 0 and 71% had 1; 33% had stable brain metastases; 94% had a mutation in the ERBB2 kinase domain and 6% had a mutation in the extracellular domain. The median number of prior regimens was 2 (range: 1 to 12); 100% of patients received prior platinum therapy, 71% received prior immunotherapy, and 44% received both in combination. Fifty percent of patients were never-smokers and 50% were former smokers; 96% of patients had adenocarcinoma histology.
Efficacy results are provided in Table 24.
Table 24: Efficacy Results for DESTINY-Lung02* Efficacy Parameter DESTINY-Lung02
N=52ORR 95% CI calculated using Clopper-Pearson method
NE=not estimableConfirmed Objective Response Rate (95% CI) 57.7% (43.2, 71.3) Complete Response 1.9% Partial Response 55.8% Duration of Response
Median, months (95% CI)†8.7 (7.1, NE) 14.4 HER2-Positive Locally Advanced or Metastatic Gastric Cancer
The efficacy of ENHERTU was evaluated in study DESTINY-Gastric01 (NCT03329690), a multicenter, open-label, randomized trial conducted in Japan and South Korea that enrolled 188 adult patients with HER2-positive (IHC 3+ or IHC 2+/ISH positive), locally advanced or metastatic gastric or GEJ adenocarcinoma who had progressed on at least two prior regimens including trastuzumab, a fluoropyrimidine- and a platinum-containing chemotherapy. HER2 expression was determined by a central lab on tissue obtained either before or after prior trastuzumab treatment. Patients were excluded for a history of treated or current ILD, a history of clinically significant cardiac disease, active brain metastases, or ECOG performance status >1.
Patients were randomized 2:1 to receive ENHERTU (N=126) 6.4 mg/kg intravenously every 3 weeks or physician's choice of chemotherapy: irinotecan monotherapy (N=55) 150 mg/m2 intravenously every 2 weeks or paclitaxel monotherapy (N=7) 80 mg/m2 intravenously weekly. Randomization was stratified by HER2 status (IHC 3+ or IHC 2+/ISH+), ECOG performance status (0 or 1), and region (Japan or South Korea). Tumor imaging assessments were performed at screening and every 6 weeks from the first treatment dose. Treatment was administered until unacceptable toxicity or disease progression. The major efficacy outcomes were ORR assessed by ICR according to RECIST v1.1 and OS in the intent-to-treat population. Additional efficacy outcomes were PFS and DOR.
The median age was 66 years (range 28 to 82); 76% were male; and 100% were Asian. All patients received a trastuzumab product. Patients had an ECOG performance status of either 0 (49%) or 1 (51%); 87% had gastric adenocarcinoma and 13% had GEJ adenocarcinoma; 76% were IHC 3+ and 23% were IHC 2+/ISH+; 65% had inoperable advanced cancer; 35% had postoperative recurrent cancer; 54% had liver metastases; 29% had lung metastases; 45% had three or more prior regimens in the locally advanced or metastatic setting. A total of 30% of patients were identified as HER2-positive using tissue obtained following prior treatment with a trastuzumab product.
Efficacy results are summarized in Table 25, and the Kaplan-Meier curve for OS is shown in Figure 8.
Table 25: Efficacy Results in DESTINY-Gastric01 Efficacy Parameter ENHERTU
N=126Irinotecan or Paclitaxel
N=62CI = confidence interval; NR = not reached - *
- OS was evaluated following a statistically significant outcome of ORR.
- †
- Median based on Kaplan-Meier estimate; 95% CI for median calculated using Brookmeyer-Crowley method
- ‡
- Based on the stratified Cox proportional hazards regression model (stratified by region)
- §
- Based on the stratified log-rank test (stratified by region)
- ¶
- Assessed by independent central review
- #
- 95% exact binomial confidence interval
- Þ
- Based on the stratified Cochran-Mantel-Haenszel test (stratified by region)
Overall Survival (OS)* Median, months (95% CI)† 12.5 (9.6, 14.3) 8.4 (6.9,10.7) Hazard ratio (95% CI)‡ 0.59 (0.39, 0.88) p-value§ 0.0097 Progression-Free Survival (PFS)¶ Median, months (95% CI)† 5.6 (4.3, 6.9) 3.5 (2.0, 4.3) Hazard ratio (95% CI)‡ 0.47 (0.31, 0.71) Confirmed Objective Response Rate (ORR)¶ n (%) 51 (40.5) 7 (11.3) 95% CI# (31.8, 49.6) (4.7, 21.9) p-valueÞ <0.0001 Complete Response n (%) 10 (7.9) 0 (0.0) Partial Response n (%) 41 (32.5) 7 (11.3) Duration of Response (DOR)¶ Median, months (95% CI)† 11.3 (5.6, NR) 3.9 (3.0, 4.9) Figure 8: Kaplan-Meier Plot of Overall Survival 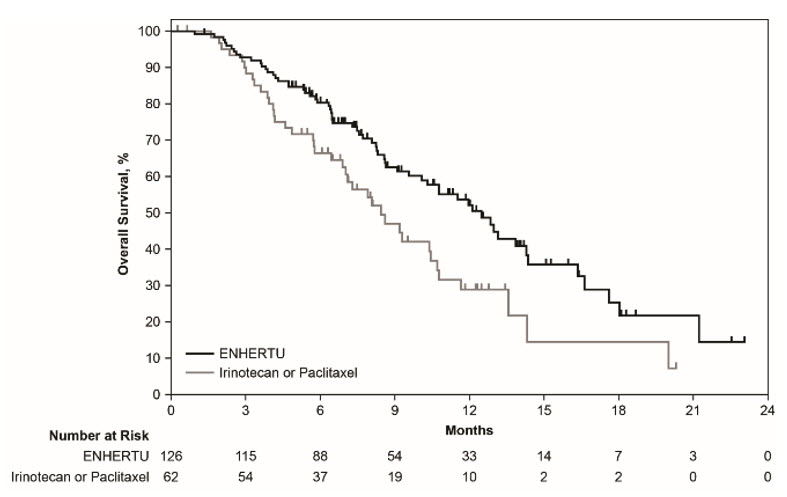 Close
Close14.5 HER2-Positive (IHC 3+) Unresectable or Metastatic Solid Tumors
The efficacy of ENHERTU was evaluated in 192 adult patients with previously treated unresectable or metastatic HER2-positive (IHC 3+) solid tumors who were enrolled in one of three multicenter trials: DESTINY-PanTumor02, DESTINY-Lung01, and DESTINY-CRC02. All three studies excluded patients with a history of ILD/pneumonitis requiring treatment with steroids or ILD/pneumonitis at screening and clinically significant cardiac disease. Patients were also excluded for active brain metastases or ECOG performance status >1. Patients received ENHERTU 5.4 mg/kg by intravenous infusion every three weeks. Treatment was administered until disease progression, death, withdrawal of consent, or unacceptable toxicity. The major efficacy outcome measure in all three of the studies was confirmed objective response rate (ORR) and an additional efficacy outcome measure was duration of response (DOR). All outcomes were assessed by independent central review (ICR) based on RECIST v1.1.
DESTINY-PanTumor02
DESTINY-PanTumor02 (NCT04482309) was a multicenter, multicohort, open-label trial that included 111 adult patients with locally advanced, unresectable, or metastatic HER2-positive (IHC 3+ by either local or central assessment) solid tumors that progressed following at least one prior systemic regimen in the advanced/metastatic setting or that had no satisfactory alternative treatment option.
The median age was 64 years (range 23 to 85); 59% were female; 58% were White, 34% were Asian, and 4% were Black or African American; 3% of patients were of Hispanic/Latino ethnicity. Patients had an ECOG performance status of either 0 (49%) or 1 (51%) at baseline. The median number of prior regimens in any treatment setting was 2.
DESTINY-Lung01
DESTINY-Lung01 (NCT03505710) was a multicenter, open-label, 2-cohort trial that included 17 patients with previously treated, unresectable, or metastatic, centrally confirmed HER2-positive (IHC 3+) NSCLC. Patients must have relapsed from or be refractory to standard treatment or have no available standard treatment.
The median age was 59 years (range 31 to 74); 59% were male; 65% were White, 18% were Asian, and 12% were Black or African American. Patients had an ECOG performance status of either 0 (12%) or 1 (88%) at baseline. The median number of prior regimens in any treatment setting was 3.
DESTINY-CRC02
DESTINY-CRC02 (NCT04744831) was a multicenter, randomized, 2-arm trial that included 64 patients with previously treated, unresectable or metastatic centrally confirmed HER2-positive (IHC 3+) colorectal cancer (CRC). Unless contraindicated, patients must have received fluoropyrimidine, oxaliplatin and irinotecan. If clinically indicated, patients must have received anti-EGFR treatment, anti-VEGF treatment and anti-PDL1 therapy.
The median age was 58 years (range 25 to 78); 53% were male; 55% were Asian and 41% were White; 1.6% of patients were of Hispanic/Latino ethnicity. Patients had an ECOG performance status of either 0 (58%) or 1 (42%) at baseline. The median number of prior regimens in any treatment setting was 4.
Efficacy results are summarized in Table 26 and Table 27.
Table 26: Efficacy Results in HER2-Positive (IHC 3+) Patients in DESTINY-PanTumor02, DESTINY-Lung01, and DESTINY-CRC02 Efficacy Parameter DESTINY-PanTumor02
N=111DESTINY-Lung01
N=17DESTINY-CRC02
N=64CI=Confidence interval
+ Denotes ongoing responseConfirmed ORR (95% CI)*† 51.4% (41.7, 61.0) 52.9% (27.8, 77.0) 46.9% (34.3, 59.8) Complete Response Rate 2.7% 5.9% 0% Partial Response Rate 48.6% 47.1% 46.9% Duration of Response* Median‡, months (range) 19.4 (1.3, 27.9+) 6.9 (4.0, 11.7+) 5.5 (1.3+, 9.7+) Table 27: Efficacy Results in HER2-positive (IHC 3+) Patients by Tumor Type in DESTINY-PanTumor02, DESTINY-Lung01, and DESTINY-CRC02 Tumor Type Patients Confirmed ORR* DOR*
RangeN % (95% CI)† (months) CI=Confidence interval, NA=Not applicable, PD=Progressive disease, PR=Partial response, SD=Stable disease
+ Denotes ongoing responseColorectal Cancer 64 46.9 (34.3, 59.8) (1.3+, 9.7+) Bladder Cancer 27 37.0 (19.4, 57.6) (2.8, 19.7+) Biliary Tract Cancer 22 45.5 (24.4, 67.8) (2.1, 22.0+) NSCLC 17 52.9 (27.8, 77.0) (4.0, 11.7+) Endometrial Cancer 16 56.3 (29.9, 80.2) (5.8, 23.7+) Ovarian Cancer 15 66.7 (38.4, 88.2) (1.3, 27.9+) Cervical Cancer 10 70.0 (34.8, 93.3) (7.2+, 25.0+) Salivary Gland Cancer 9 66.7 (29.9,92.5) (5.6, 20.1) Pancreatic Cancer 5 0 (0, 52.2) NA Oropharyngeal Neoplasm 1 PR 15.3 Vulvar Cancer 1 PR 2.6 Extramammary Paget's Disease 1 PR 19.4 Lacrimal Gland Cancer 1 PR 19.8+ Lip and/or Oral Cavity Cancer 1 SD NA Esophageal Adenocarcinoma 1 PR 2.8 Esophageal Squamous Cell Carcinoma 1 PD NA -
15 REFERENCESOSHA Hazardous Drugs. OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied/Storage - ENHERTU (fam-trastuzumab deruxtecan-nxki) for injection is a white to yellowish white lyophilized powder supplied as: Carton ContentsNDC - One 100 mg single-dose ...
How Supplied/Storage
ENHERTU (fam-trastuzumab deruxtecan-nxki) for injection is a white to yellowish white lyophilized powder supplied as:
Carton Contents NDC One 100 mg single-dose vial NDC 65597-406-01 Store vials in a refrigerator at 2ºC to 8ºC (36ºF to 46ºF) in the original carton to protect from light until time of reconstitution. Do not freeze. Do not shake the reconstituted or diluted solution [see Dosage and Administration (2.4)].
CloseSpecial Handling
ENHERTU (fam-trastuzumab deruxtecan-nxki) is a hazardous drug. Follow applicable special handling and disposal procedures.1
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Interstitial Lung Disease - Inform patients of the risks of severe or fatal ILD. Advise patients to contact ...
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Interstitial Lung Disease
- Inform patients of the risks of severe or fatal ILD. Advise patients to contact their healthcare provider immediately for any of the following: cough, shortness of breath, fever, or other new or worsening respiratory symptoms [see Warnings and Precautions (5.1)].
Neutropenia
- Advise patients of the possibility of developing neutropenia and to immediately contact their healthcare provider should they develop a fever, particularly in association with any signs of infection [see Warnings and Precautions (5.2)].
Left Ventricular Dysfunction
- Advise patients to contact their healthcare provider immediately for any of the following: new onset or worsening shortness of breath, cough, fatigue, swelling of ankles/legs, palpitations, sudden weight gain, dizziness, loss of consciousness [see Warnings and Precautions (5.3)].
Embryo-Fetal Toxicity
- Inform female patients of the potential risk to a fetus. Advise female patients to contact their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.4), Use in Specific Populations (8.1)].
- Advise females of reproductive potential to use effective contraception during treatment with ENHERTU and for 7 months after the last dose [see Use in Specific Populations (8.3)].
- Advise male patients with female partners of reproductive potential to use effective contraception during treatment with ENHERTU and for 4 months after the last dose [see Use in Specific Populations (8.3)].
Lactation
- Advise women not to breastfeed during treatment and for 7 months after the last dose of ENHERTU [see Use in Specific Populations (8.2)].
CloseInfertility
- Advise males of reproductive potential that ENHERTU may impair fertility [see Use in Specific Populations (8.3)].
-
SPL UNCLASSIFIED SECTIONManufactured by: Daiichi Sankyo, Inc., Basking Ridge, NJ 07920 - U.S. License No. 2128 - Marketed by: Daiichi Sankyo, Inc., Basking Ridge, NJ 07920 and AstraZeneca Pharmaceuticals LP, Wilmington, DE ...
Manufactured by:
Daiichi Sankyo, Inc., Basking Ridge, NJ 07920U.S. License No. 2128
Marketed by:
Daiichi Sankyo, Inc., Basking Ridge, NJ 07920 and AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850ENHERTU® is a registered trademark of Daiichi Sankyo Company, Ltd.
© 2025 Daiichi Sankyo Co., Ltd.USPI-ENH-C18.1-0125-r009
Close -
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration.Revised: 01/2025 Medication Guide - ENHERTU® (en-HER-too) (fam-trastuzumab deruxtecan-nxki) for ...Close
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 01/2025 Medication Guide
ENHERTU® (en-HER-too)
(fam-trastuzumab deruxtecan-nxki) for injectionWhat is the most important information I should know about ENHERTU?
ENHERTU can cause serious side effects, including:-
Lung problems that may be severe, life-threatening or that may lead to death. If you develop lung problems your healthcare provider may treat you with corticosteroid medicines. Tell your healthcare provider right away if you get any of the following signs and symptoms:
- cough
- trouble breathing or shortness of breath
- fever
- other new or worsening breathing symptoms (such as chest tightness, wheezing)
- Low white blood cell count (neutropenia). Low white blood cell counts are common with ENHERTU and can sometimes be severe. Your healthcare provider will check your white blood cell counts before starting ENHERTU and before starting each dose. Tell your healthcare provider right away if you develop any signs or symptoms of an infection or have fever or chills during treatment with ENHERTU.
- Heart problems that may affect your heart's ability to pump blood. Your healthcare provider will check your heart function before starting treatment with ENHERTU. Tell your healthcare provider right away if you get any of the following signs and symptoms:
- new or worsening shortness of breath
- coughing
- feeling tired
- swelling of your ankles or legs
- irregular heartbeat
- sudden weight gain
- dizziness or feeling light-headed
- loss of consciousness
Your healthcare provider will check you for these side effects during your treatment with ENHERTU. Your healthcare provider may reduce your dose, delay treatment or completely stop treatment with ENHERTU if you have severe side effects. -
Harm to your unborn baby. Tell your healthcare provider right away if you become pregnant or think you might be pregnant during treatment with ENHERTU.
- If you are able to become pregnant, your healthcare provider should do a pregnancy test before you start treatment with ENHERTU.
- Females who are able to become pregnant should use effective birth control (contraception) during treatment with ENHERTU and for 7 months after the last dose.
- Males who have female partners that are able to become pregnant should use effective birth control (contraception) during treatment with ENHERTU and for 4 months after the last dose.
What is ENHERTU?
ENHERTU is a prescription medicine used to treat adults who have:- human epidermal growth factor receptor 2 (HER2)-positive breast cancer that cannot be removed by surgery or that has spread to other parts of the body (metastatic), and who have received a prior anti-HER2 breast cancer treatment:
- for metastatic disease, or
- have breast cancer that has come back during or within 6 months of completing treatment for their early-stage breast cancer.
- HER2-low breast cancer that cannot be removed by surgery or that has spread to other parts of the body (metastatic), and who have received a prior chemotherapy
- for metastatic disease, or
- whose disease has returned during or within 6 months of completing adjuvant chemotherapy (after surgery). Your healthcare provider will perform a test to make sure ENHERTU is right for you.
- Hormone receptor (HR)-positive, HER2-low or HR-positive, HER2-ultralow, breast cancer that cannot be removed by surgery or that has spread to other parts of the body (metastatic), and who have received one or more endocrine treatments for metastatic disease.
Your healthcare provider will perform a test to make sure ENHERTU is right for you.
- non-small cell lung cancer (NSCLC) that has a certain mutation in the HER2 gene and cannot be removed by surgery or has spread to other parts of your body (metastatic), and who have received a prior treatment. Your healthcare provider will perform a test to make sure ENHERTU is right for you.
- stomach cancer called gastric or gastroesophageal junction (GEJ) adenocarcinoma that is HER2-positive and has spread to areas near your stomach (locally advanced) or that has spread to other parts of your body (metastatic), and who have received a prior trastuzumab-based regimen.
- solid tumors that are HER2-positive (IHC 3+) and that cannot be removed by surgery or have spread to other parts of your body (metastatic), and who have received a prior treatment and have no other satisfactory treatment options. Your healthcare provider will perform a test to make sure ENHERTU is right for you.
Before you receive ENHERTU, tell your healthcare provider about all of your medical conditions, including if you: - have lung or breathing problems
- have signs or symptoms of an infection
- have or have had any heart problems
- are breastfeeding or plan to breastfeed. It is not known if ENHERTU passes into your breast milk. Do not breastfeed during treatment with ENHERTU and for 7 months after the last dose.
How will I receive ENHERTU? - You will receive ENHERTU into your vein through an intravenous (IV) line by your healthcare provider.
- ENHERTU is given 1 time every three weeks (21-day treatment cycle).
- Your healthcare provider will decide how many treatments you need.
- Your healthcare provider will give you medicines before your infusion to help prevent nausea and vomiting.
- Your healthcare provider may slow down or temporarily stop your infusion of ENHERTU if you have an infusion-related reaction, or permanently stop ENHERTU if you have severe infusion reactions.
- If you miss a planned dose of ENHERTU, call your healthcare provider right away to schedule an appointment. Do not wait until the next planned treatment cycle.
What are the possible side effects of ENHERTU?
ENHERTU can cause serious side effects. See "What is the most important information I should know about ENHERTU?"
The most common side effects of ENHERTU, when used in people with metastatic breast cancer, HER2-mutant non-small cell lung cancer, and other HER2-positive solid tumors include:- low white blood cell counts
- nausea
- low red blood cell counts
- feeling tired
- low platelet counts
- increased liver function tests
- vomiting
- hair loss
- constipation
- low levels of blood potassium
- decreased appetite
- diarrhea
- muscle or bone pain
The most common side effects of ENHERTU, when used in people with HER2-positive gastric or GEJ adenocarcinoma, include: - low red blood cell counts
- low white blood cell counts
- low platelet counts
- nausea
- decreased appetite
- increased liver function tests
- feeling tired
- diarrhea
- low levels of blood potassium
- vomiting
- constipation
- fever
- hair loss
ENHERTU may cause fertility problems in males, which may affect the ability to father children. Talk to your healthcare provider if you have concerns about fertility.
These are not all of the possible side effects of ENHERTU. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about the safe and effective use of ENHERTU.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or healthcare provider for information about ENHERTU that is written for healthcare professionals.What are the ingredients in ENHERTU?
Active Ingredient: fam-trastuzumab deruxtecan-nxki.
Inactive Ingredients: L-histidine, L-histidine hydrochloride monohydrate, polysorbate 80, and sucrose.
Manufactured by: Daiichi Sankyo, Inc., Basking Ridge, NJ 07920
U.S. License No. 2128
Marketed by: Daiichi Sankyo, Inc., Basking Ridge, NJ 07920 and AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850
ENHERTU® is a registered trademark of Daiichi Sankyo Company, Ltd.
© 2025 Daiichi Sankyo Co., Ltd.
USMG-ENH-C18.1-0125-r008
For more information, call 1-877-437-7763 or go to https://www.ENHERTU.com. -
Lung problems that may be severe, life-threatening or that may lead to death. If you develop lung problems your healthcare provider may treat you with corticosteroid medicines. Tell your healthcare provider right away if you get any of the following signs and symptoms:
-
PRINCIPAL DISPLAY PANEL - 100 mg Vial CartonNDC 65597-406-01 - Rx only - ENHERTU® (fam-trastuzumab deruxtecan-nxki) For Injection - 100 mg per vial - For Intravenous Infusion Only - Dispense the enclosed Medication Guide to each ...
NDC 65597-406-01
Rx onlyENHERTU®
(fam-trastuzumab deruxtecan-nxki)For Injection
100 mg per vial
For Intravenous Infusion Only
Dispense the enclosed Medication Guide to each patient.
Reconstitute and Dilute prior to administration
Single-Dose Vial
Discard Unused PortionHazardous Drug
KEEP REFRIGERATED
1 vial
Daiichi-Sankyo
AstraZenecaClose
-
INGREDIENTS AND APPEARANCEProduct Information
ENHERTU fam-trastuzumab deruxtecan-nxki injection, powder, lyophilized, for solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65597-406 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRASTUZUMAB DERUXTECAN (UNII: 5384HK7574) (TRASTUZUMAB DERUXTECAN - UNII:5384HK7574) TRASTUZUMAB DERUXTECAN 100 mg in 5 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) HISTIDINE MONOHYDROCHLORIDE MONOHYDRATE (UNII: X573657P6P) SUCROSE (UNII: C151H8M554) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65597-406-01 1 in 1 CARTON 12/20/2019 1 5 mL in 1 VIAL, SINGLE-DOSE; Type 6: Drug/Biologic Combination Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761139 12/20/2019 Labeler - Daiichi Sankyo Inc. (068605067)
CloseRegistrant - Baxter Oncology GmbH (344276063)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
ENHERTU- fam-trastuzumab deruxtecan-nxki injection, powder, lyophilized, for solution
Number of versions: 15
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Feb 3, 2025 | 20 (current) | download |
| Apr 18, 2024 | 18 | download |
| Feb 28, 2024 | 17 | download |
| Feb 6, 2024 | 16 | download |
| Feb 14, 2023 | 15 | download |
| Nov 15, 2022 | 14 | download |
| Nov 7, 2022 | 13 | download |
| Sep 22, 2022 | 12 | download |
| Aug 24, 2022 | 11 | download |
| Aug 18, 2022 | 9 | download |
| May 16, 2022 | 7 | download |
| Nov 9, 2021 | 6 | download |
| Jan 21, 2021 | 5 | download |
| Jan 23, 2020 | 4 | download |
| Dec 24, 2019 | 3 | download |
RxNorm
ENHERTU- fam-trastuzumab deruxtecan-nxki injection, powder, lyophilized, for solution
Get Label RSS Feed for this Drug
ENHERTU- fam-trastuzumab deruxtecan-nxki injection, powder, lyophilized, for solution
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=7e67e73e-ddf4-4e4d-8b50-09d7514910b6
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
ENHERTU- fam-trastuzumab deruxtecan-nxki injection, powder, lyophilized, for solution
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 65597-406-01 |