Label: VTAMA- tapinarof cream
- NDC Code(s): 81672-5051-1
- Packager: Dermavant Sciences, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VTAMA - ®cream safely and effectively. See full prescribing information for VTAMA. VTAMA (tapinarof) cream, 1%, for topical ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Plaque Psoriasis - VTAMA - ®cream is indicated for the topical treatment of plaque psoriasis in adults. 1.2 Atopic Dermatitis - VTAMA cream is indicated for the topical treatment of ...
-
2 DOSAGE AND ADMINISTRATIONApply a thin layer of VTAMA cream to affected areas once daily. Wash hands after application, unless VTAMA cream is for treatment of the hands. VTAMA cream is not for oral, ophthalmic, or ...
-
3 DOSAGE FORMS AND STRENGTHSCream, 1% Each gram of VTAMA cream contains 10 mg of tapinarof in a white to off-white cream.
-
4 CONTRAINDICATIONSNone.
-
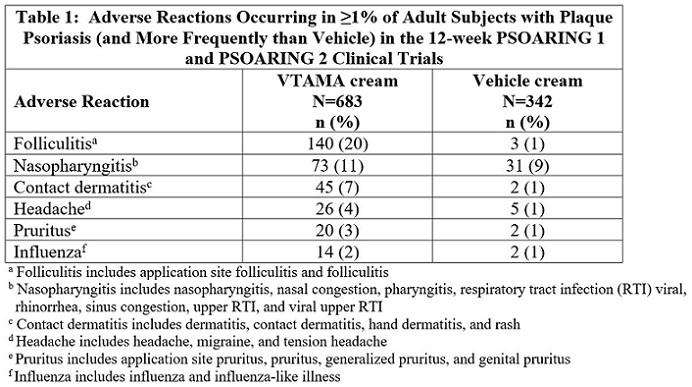
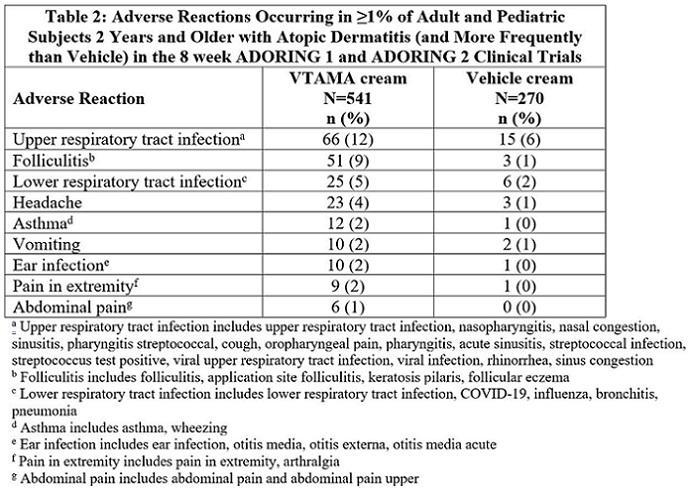
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - The available data on VTAMA cream use in pregnant women are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage, or other ...
-
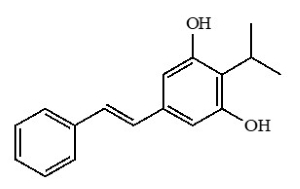
11 DESCRIPTIONVTAMA (tapinarof) cream contains tapinarof as the active ingredient. Tapinarof is an aryl hydrocarbon receptor agonist. Tapinarof is a white to pale brown powder. Chemically, tapinarof is 3 ...
-
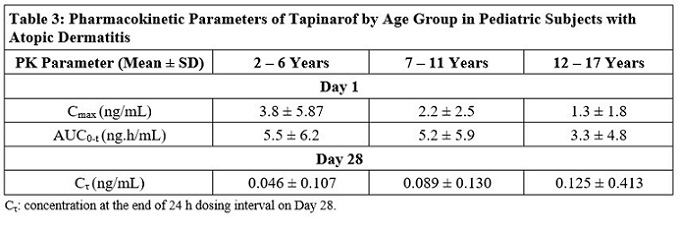
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Tapinarof is an aryl hydrocarbon receptor (AhR) agonist. The specific mechanisms by which VTAMA cream exerts its therapeutic action in psoriasis patients are ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term carcinogenicity studies were conducted in mice (daily topical administration at doses of 0.5, 1.5 and 3% tapinarof cream) and ...
-
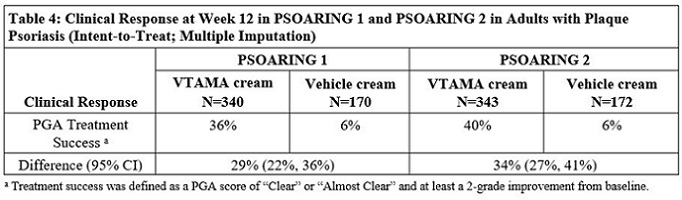
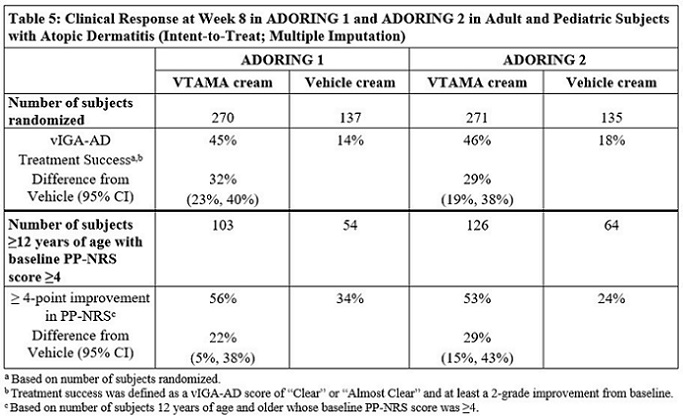
14 CLINICAL STUDIES14.1 Plaque Psoriasis - Two multicenter, randomized, double-blind, vehicle-controlled trials were conducted to evaluate the safety and efficacy of VTAMA cream for the treatment of adults with ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGVTAMA (tapinarof) cream, 1% is a white to off-white cream. Each gram of VTAMA cream contains 10 mg of tapinarof. It is supplied in the following size: 60 g laminated tubes: NDC ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Administration Instructions - • Apply VTAMA cream once daily to psoriasis skin lesions only and avoid ...
- INFORMATION FOR PATIENTS
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - VTAMA - ®(Vee-TAM-uh) (tapinarof) cream, for topical use - Important information: VTAMA cream is for use on the skin (topical use) only.Do not use VTAMA cream ...
-
Package Label60 gram carton - 60 gram tube
-
INGREDIENTS AND APPEARANCEProduct Information