Label: FILSPARI- sparsentan tablet, film coated
- NDC Code(s): 68974-200-30, 68974-400-30
- Packager: Travere Therapeutics, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated September 4, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use FILSPARI® safely and effectively. See full prescribing information for FILSPARI®. FILSPARI® (sparsentan) tablets, for oral ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: HEPATOTOXICITY and EMBRYO-FETAL TOXICITY
Because of the risks of hepatotoxicity and birth defects, FILSPARI is available only through a restricted program called the FILSPARI REMS. Under the FILSPARI REMS, prescribers, patients, and pharmacies must enroll in the program [see Warnings and Precautions (5.1, 5.2, 5.3)].
Hepatotoxicity
Some Endothelin Receptor Antagonists (ERAs) have caused elevations of aminotransferases, hepatotoxicity, and liver failure. In clinical studies, elevations in aminotransferases (ALT or AST) of at least 3-times the Upper Limit of Normal (ULN) have been observed in up to 3.5% of FILSPARI-treated patients, including cases confirmed with rechallenge.
Measure transaminases and bilirubin before initiating treatment and monthly for the first 12 months, and then every 3 months during treatment. Interrupt treatment and closely monitor patients who develop aminotransferase elevations more than 3-times ULN [see Dosage and Administration (2.3, 2.5), Warnings and Precautions (5.1)].
FILSPARI should generally be avoided in patients with elevated aminotransferases (>3-times ULN) at baseline because monitoring for hepatotoxicity may be more difficult and these patients may be at increased risk for serious hepatotoxicity [see Dosage and Administration (2.3, 2.5), Warnings and Precautions (5.1)].
Embryo-Fetal Toxicity
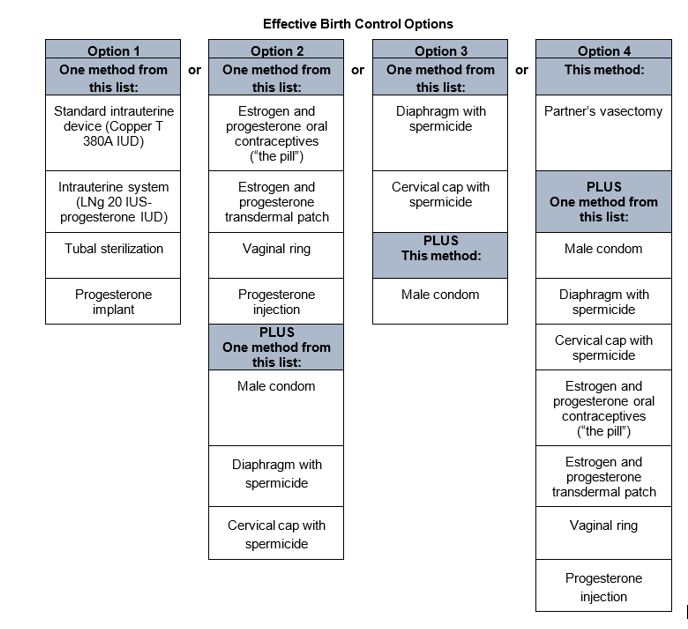
FILSPARI can cause major birth defects if used by pregnant patients based on animal data [see Warnings and Precautions (5.2), Use in Specific Populations (8.1)]. Therefore, pregnancy testing is required before the initiation of treatment, during treatment, and one month after discontinuation of treatment with FILSPARI. Patients who can become pregnant must use effective contraception before the initiation of treatment, during treatment, and for one month after discontinuation of treatment with FILSPARI [see Dosage and Administration (2.2), Contraindications (4), Warnings and Precautions (5.2), Use in Specific Populations (8.1, 8.3)].
Close -
1 INDICATIONS AND USAGEFILSPARI is indicated to slow kidney function decline in adults with primary immunoglobulin A nephropathy (IgAN) who are at risk for disease progression.
-
2 DOSAGE AND ADMINISTRATION2.1 General Considerations - Prior to initiating treatment with FILSPARI, discontinue use of renin-angiotensin-aldosterone system (RAAS) inhibitors and endothelin receptor antagonists ...
-
3 DOSAGE FORMS AND STRENGTHSFILSPARI is supplied as film-coated, modified oval, white to off-white tablets debossed on one side and plain on the other in the following strengths: 200 mg debossed with “105” 400 mg ...
-
4 CONTRAINDICATIONSUse of FILSPARI is contraindicated in patients who are pregnant [see Dosage and Administration (2.2), Warnings and Precautions (5.2), Use in Specific Populations (8.1)]. Do not coadminister ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hepatotoxicity - .Elevations in ALT or AST of at least 3-fold ULN have been observed in up to 3.5% of FILSPARI-treated patients, including cases confirmed with rechallenge [see Adverse ...
-
6 ADVERSE REACTIONSClinically significant adverse reactions that appear in other sections of the label include: Hepatotoxicity [see Warnings and Precautions (5.1)] Embryo-Fetal Toxicity [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Renin-Angiotensin System Inhibitors and ERAs - Do not coadminister FILSPARI with ARBs, ERAs, or aliskiren [see Dosage and Administration (2.1), Contraindications (4)]. Combined use of these ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on data from animal reproductive toxicity studies, FILSPARI can cause fetal harm, including birth defects and fetal death, when administered to a pregnant ...
-
10 OVERDOSAGEThere is no experience with overdose with FILSPARI. Sparsentan has been given in doses up to 1600 mg/day in healthy volunteers, or up to 400 mg/day in IgAN patients. Overdose of FILSPARI may ...
-
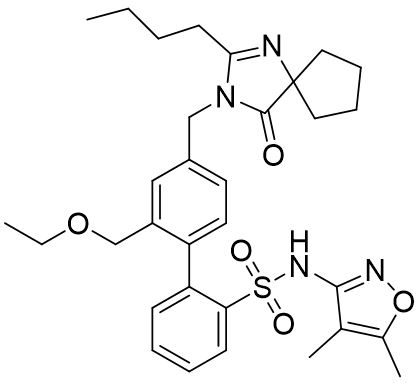
11 DESCRIPTIONFILSPARI (sparsentan) is an endothelin and angiotensin II receptor antagonist. The chemical name of sparsentan is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Sparsentan is a single molecule with antagonism of the endothelin type A receptor (ETAR) and the angiotensin II type 1 receptor (AT1R). Sparsentan has high affinity ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility - Carcinogenesis: In the two-year rat carcinogenicity study, there was no evidence of increased incidence of neoplasia in male rats ...
-
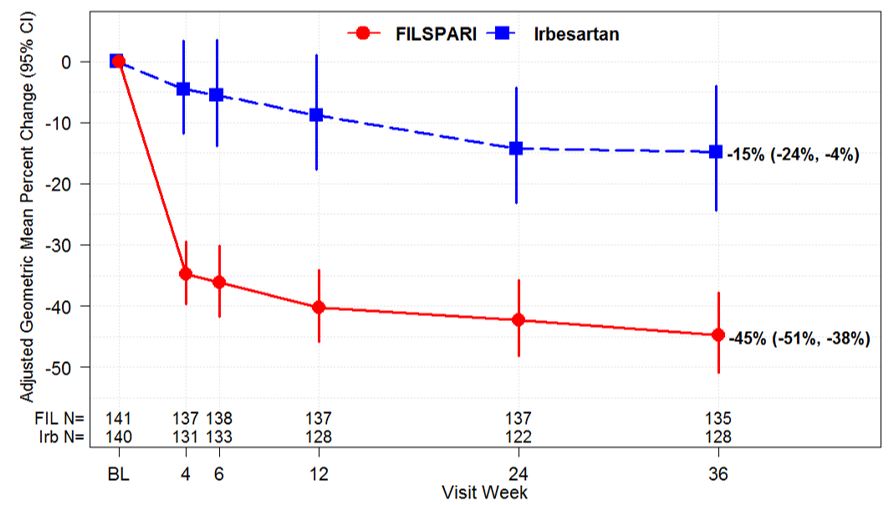
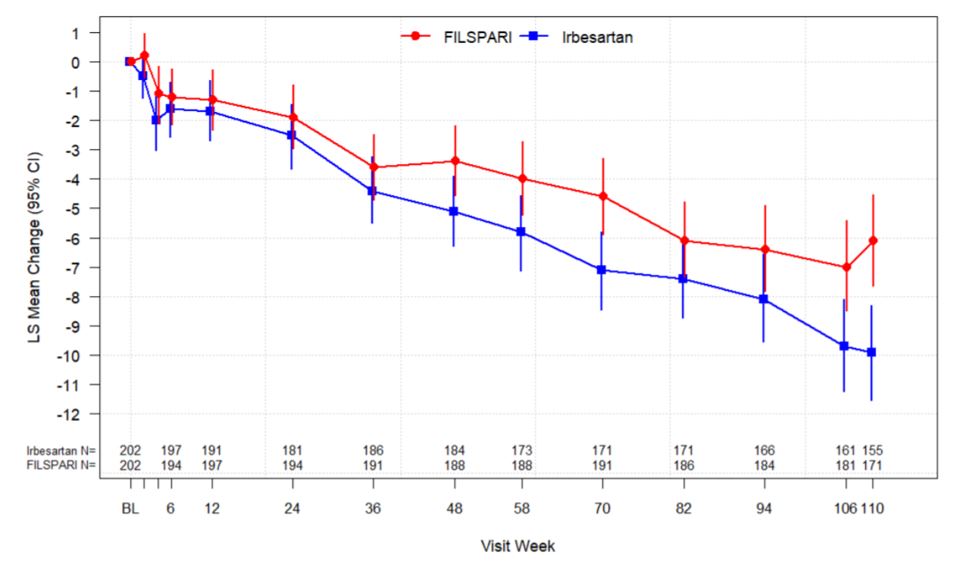
14 CLINICAL STUDIESThe effect of FILSPARI on proteinuria and kidney function (estimated glomerular filtration rate, eGFR) was assessed in a randomized, double-blind, active-controlled, multicenter, global ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGFILSPARI is supplied in bottles of 30 film-coated tablets. 200 mg tablets are film-coated, modified oval, white to off-white, debossed with “105” on one side and plain on the other side ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients to read the FDA-approved patient labeling (Medication Guide). Restricted access - Advise the patient that FILSPARI is only available through a restricted access program called ...
-
MEDICATION GUIDEMedication Guide - FILSPARI® (fil spah ree) (sparsentan) tablets - What is the most important information I should know about FILSPARI? FILSPARI is only available through the ...
-
PRINCIPAL DISPLAY PANEL - 200 mg Tablet Bottle LabelNDC 68974-200-30 - 30 Tablets - FILSPARI™ (sparsentan) tablets - 200 mg - Rx Only - Manufactured for: Travere Therapeutics, Inc., San Diego, CA 92130 - Manufactured by: Catalent - Kansas City, MO 64137
-
PRINCIPAL DISPLAY PANEL - 400 mg Tablet LabelNDC 68974-400-30 - 30 Tablets - FILSPARI™ (sparsentan) tablets - 400 mg - Rx Only - Manufactured for: Travere Therapeutics, Inc., San Diego, CA 92130 - Manufactured by: Catalent - Kansas City, MO 64137
-
INGREDIENTS AND APPEARANCEProduct Information