Label: PROVENGE- sipuleucel-t injection
- NDC Code(s): 30237-8900-5, 30237-8900-6
- Packager: Dendreon Pharmaceuticals LLC
- Category: CELLULAR THERAPY
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated October 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PROVENGE - ® (sipuleucel-T) safely and effectively. See full prescribing information for PROVENGE. PROVENGE - ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE PROVENGE - ® (sipuleucel-T) is an autologous cellular immunotherapy indicated for the treatment of asymptomatic or minimally symptomatic metastatic castrate-resistant ...
-
2 DOSAGE AND ADMINISTRATION For autologous use only. For intravenous use only. 2.1 Dose - Each dose of PROVENGE contains a minimum of 50 million autologous CD54 - + cells activated with ...
-
3 DOSAGE FORMS AND STRENGTHS Each dose of PROVENGE contains a minimum of 50 million autologous CD54 - + cells activated with PAP-GM-CSF, suspended in 250 mL of Lactated Ringer's Injection, USP ...
-
4 CONTRAINDICATIONS None.
-
5 WARNINGS AND PRECAUTIONS 5.1 Acute Infusion Reactions - Acute infusion reactions (reported within 1 day of infusion) may occur and include nausea, vomiting, fatigue, fever, rigor or chills, respiratory events (dyspnea ...
-
6 ADVERSE REACTIONS The most common adverse reactions reported in clinical trials (≥ 15% of patients receiving PROVENGE) were chills, fatigue, fever, back pain, nausea, joint ache, and headache. 6.1 Clinical ...
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS 8.5 Geriatric - In controlled clinical trials, 72.9% of patients (438 of 601) in the PROVENGE group were ≥ 65 years of age. There were no apparent differences in the safety of PROVENGE between ...
-
10 OVERDOSAGE Each PROVENGE infusion comprises the maximum number of cells that can be manufactured from a single leukapheresis procedure. The number of cells in PROVENGE does not exceed the number of cells ...
-
11 DESCRIPTION PROVENGE (sipuleucel-T) is an autologous cellular immunotherapy available as a suspension for intravenous infusion. PROVENGE consists of autologous peripheral blood mononuclear cells, including ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - PROVENGE is classified as an autologous cellular immunotherapy. While the precise mechanism of action is unknown, PROVENGE is designed to induce an immune response ...
- 13 NONCLINICAL TOXICOLOGY
-
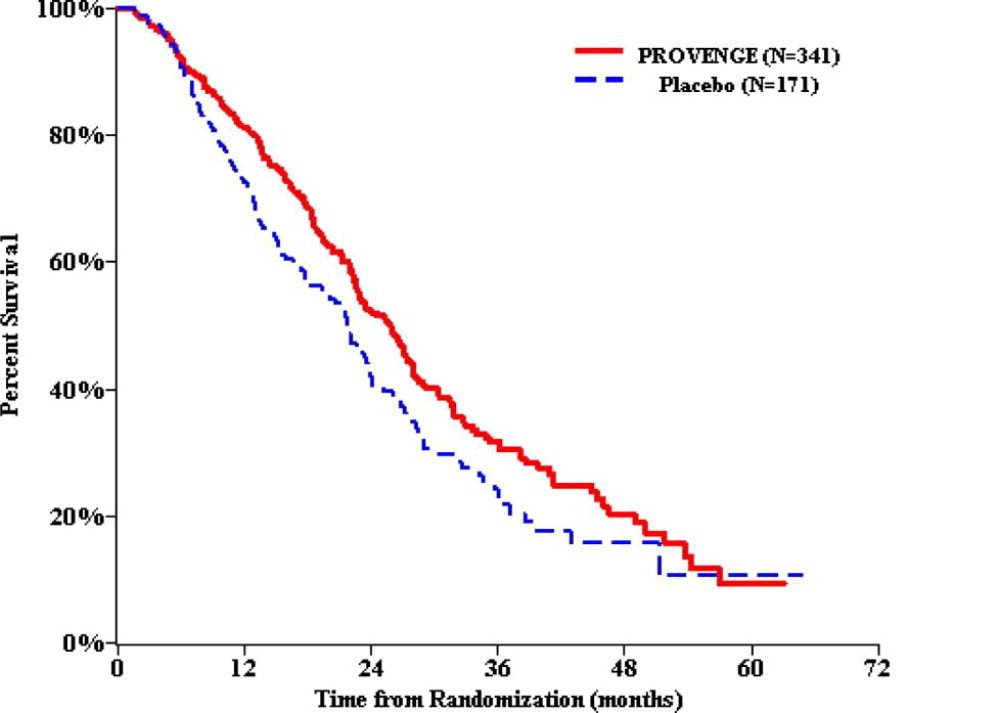
14 CLINICAL STUDIES The effect of PROVENGE on patients with metastatic castrate-resistant (hormone-refractory) prostate cancer was studied in three similar randomized, double-blind, placebo-controlled, multicenter ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING PROVENGE is a 250 mL suspension containing a minimum of 50 million autologous CD54 - + cells activated with PAP-GM-CSF in Lactated Ringer's Injection, USP. It is supplied in ...
-
17 PATIENT COUNSELING INFORMATION Advise the patient to read the FDA-approved patient labeling ( Patient Information). Inform the patient or caregiver about the ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - PROVENGE® (sipuleucel-T) This leaflet is designed to help you understand treatment with PROVENGE (pronounced PROH-venj). The more you understand your treatment, the better ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel – Carton Label - NDC 30237-8900-6 - sipuleucel-T - PROVENGE - ® RX ONLY FOR AUTOLOGOUS USE ONLY - No U.S. standard of potency - CONTENTS: A minimum of 50 million ...
-
PRINCIPAL DISPLAY PANELPrincipal Display Panel – Bag Label - NDC 30237-8900-5 - sipuleucel-T - PROVENGE - ® RX ONLY FOR AUTOLOGOUS USE ONLY - No U.S. standard of potency - CONTENTS: A minimum of 50 million ...
-
PRINCIPAL DISPLAY PANELPackage Label 2 - sipuleucel-T 250 mL Lot XXXXXX-X - Store Refrigerated 2-8°C DO NOT FREEZE - Expiration Date: DD-MMM-YYYY - Expiration Time: XX:XX Time Zone: First Name MI: Last Name: Date of ...
-
INGREDIENTS AND APPEARANCEProduct Information