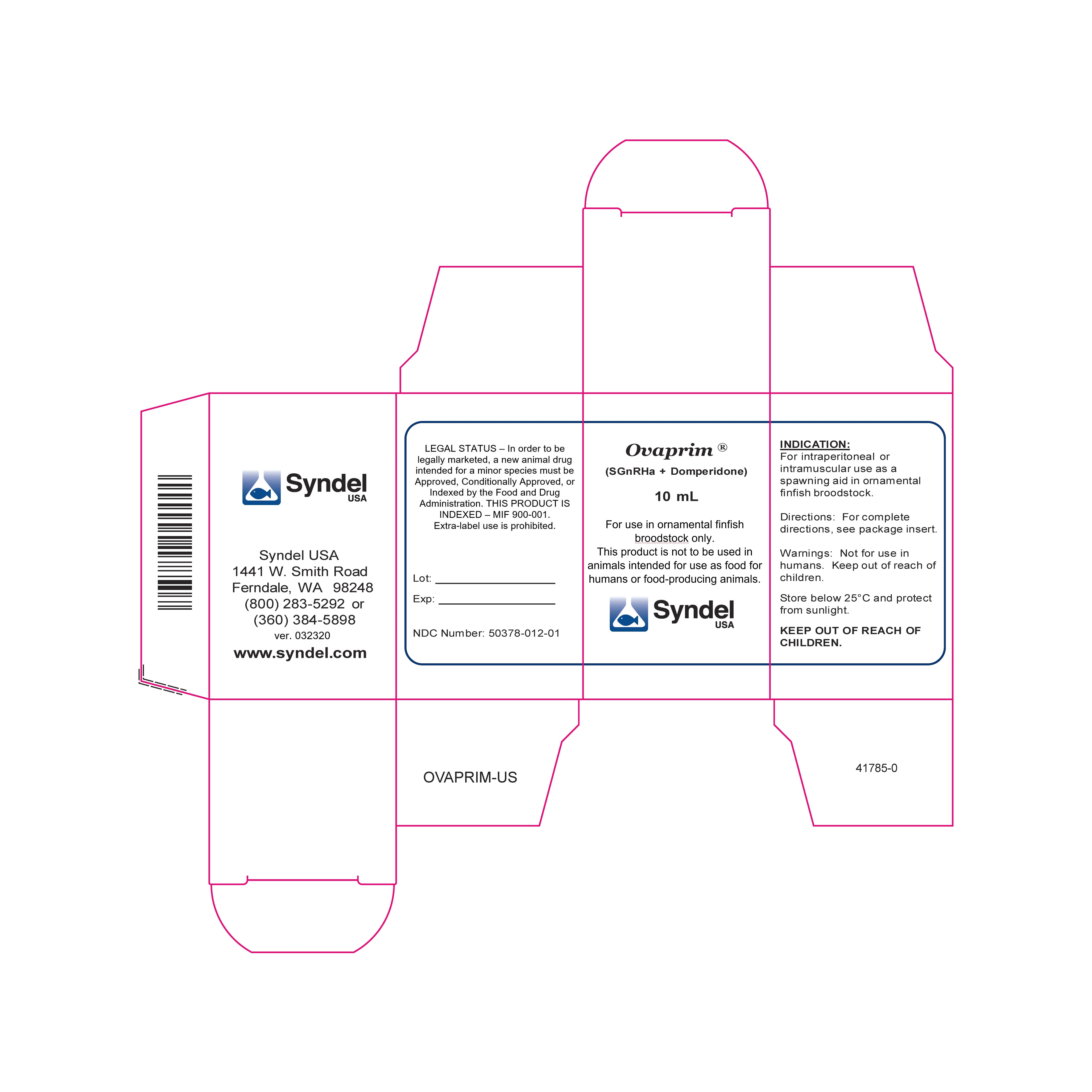
Label: OVAPRIM- sgnrha and domperidone injection, solution
- NDC Code(s): 50378-012-01
- Packager: Western Chemical Inc.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Legally Marketed Unapproved New Animal Drugs for Minor Species
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated August 12, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Note---In order to be legally marketed, an animal drug product intended for a minor species must be Approved, Conditionally Approved, or Indexed by the FDA. THIS PRODUCT IS INDEXED.
It is a violation of federal law to use this product in a manner other than as directed in the labeling.
As used on this label, the words “ornamental fish” refer to a wide variety of finfish species maintained primarily for their appearance and do not include any fish that are consumed by humans or food-producing animals.
NET CONTENTS: 10 ml
DESCRIPTION:Ovaprim® is a liquid peptide preparation that contains an analog of salmon gonadotropin releasing hormone (sGnRHa) and a brain neurotransmitter (dopamine) inhibitor. The sGnRHa in Ovaprim® elicits the release of stored gonadotropins from the pituitary. The dopamine inhibitor (Domperidone) serves to remove other inhibition of GnRH release. Release of stored pituitary gonadotropins may aid spawning by stimulating ovulation and spermiation in sexually mature fish.
INDICATION: For intraperitoneal or intramuscular use as a spawning aid in ornamental finfish broodstock.
- PRECAUTIONS
-
WARNINGS
WARNING:
Care should be taken to avoid accidental contact or self-injection. In the event of accidental self-injection, seek medical advice immediately. Use in a well ventilated area. Wear gloves, goggles and suitable protective clothing. Not for use in humans. Keep out of the reach of children.
INHALATION: May be harmful. If breathing becomes difficult, move to fresh air, and contact a physician.
INGESTION: May be harmful. If the person is conscious, wash out mouth with copious amounts of water. Contact a physician.
EYE CONTACT: May be harmful. In case of contact, flush with copious amounts of water for at least 15 minutes. Assure adequate flushing by separating eyelids with fingers. Contact a physician.
SKIN CONTACT: May be harmful. In case of contact, flush with copious amounts of water for at least 15 minutes. Remove contaminated clothing and wash before re-using. Contact a physician.
OTHER HEALTH INFORMATION: The toxicological properties of sGnRHa have not been thoroughly investigated. The actions are similar to luteinizing hormone releasing hormone (LHRH, Gonadotropin releasing hormone, GnRH) in humans. LHRH is the key mediator in the liberation of the pituitary gonadotropins, luteinizing hormone (LH) and follicle stimulating hormone (FSH). LHRH may modify reproductive ability by influencing plasma gonadotropin levels and concomitantly gonadal steroid levels.
To obtain a product Safety Data Sheet, call Syndel USA at (360) 384-5898. -
DOSAGE & ADMINISTRATION
DIRECTIONS FOR USE:
Ovaprim® is packaged ready for use in liquid form. Precise amounts for individual injections should be withdrawn directly from the bottle into a syringe. Due to the viscosity of the product, use of syringes with Luer Lock tips or permanently attached needles is strongly recommended. Use of syringes with slip tips is not recommended since needles could inadvertently eject under pressure.CLEANLINESS: Syringes/needles should be sterile prior to use.
LOADING: Withdraw only enough solution from the bottle of Ovaprim® Injectable Solution as will be required for the weight of the fish. With the needle pointed upward and directed away from your face, squeeze the syringe gently to expel any trapped air.
INJECTION: Hold the fish firmly and insert the needle into the abdominal cavity/belly between the pelvic fin and the vent or into the muscle on either side of the dorsal fin. For intra-abdominal injection in smaller species, it may be helpful to secure the fish on a soft wet surface such as a sponge, and to place the fish on its side and inject into the rear abdominal cavity to avoid contact with internal organs. Inject Ovaprim® carefully. After injection, gently place the fish into a container of clean aerated water.
SEDATION FOR HANDLING AND RECOVERY: If necessary, sedate broodstock prior to Ovaprim® injection.
DOSE:
A general dose of Ovaprim® is 0.5 ml per kilogram of bodyweight. This dose may vary among finfish species and physiological state of individual animals. Environment and temperature also play a significant role in the reproductive process and may affect dose and timing. For most species, only a single dose of Ovaprim® is required; but treatment of fish with a single dose of Ovaprim® is only effective in fish that are within or near their natural spawning season. For other species, a split dose may be needed. For split dosing, a loading dose of 10% of the total dose should be injected, followed by injection of the remaining 90% of the total dose at least 6 hours later. In warm water species, ovulation may occur in as little as 4 hours post treatment, so fish should be monitored accordingly. Signs of ovulation/spermiation may include a noticeable swelling and “softening” of the abdomen; presence of eggs in the water; the ability to easily express eggs from the female and milt from males; and when sexes are held together in the same tank, onset of spawning behavior (e.g., males “chasing” females). However, it is important to note that the interval between treatment and the onset of ovulation is variable between species and temperature, and treated fish should be disturbed as little as possible to reduce stress during final maturation. Males may respond in shorter periods of time than females of the same species.DOSE CALCULATION:
The dose is calculated based on the weight of the fish.
FISH WEIGHT x OVAPRIM® DOSAGE = AMOUNT OF INJECTION
Examples: 1) 0.2 kg 0.5 ml/kg 0.1 ml
2) 50 gm 0.5 µl/gm 25 µl
Use of a needle guard to limit the penetration of the needle into the fish may be helpful, and use of a tuberculin or similar syringe is also recommended for accurate dosing in small fish and to minimize trauma at the injection site. Intramuscular injection may result in bleeding at the injection site. Swelling, ulceration, whiteness, redness, or hypopigmentation may also be observed at the injection site. - STORAGE AND HANDLING
-
SPL UNCLASSIFIED SECTION
DISPOSAL:
Contact your State Environmental Control Agency, or the Hazardous Waste Representative at the nearest EPA Regional Office for guidance pertaining to disposal of unused product.
QUESTIONS/COMMENTS? For technical assistance, call Syndel USA at (360) 384-5898. To report an adverse event, call Syndel USA at (360) 384-5898, or FDA at 1-888-FDA-VETS.Syndel USA
1441 W. Smith Road
Ferndale, WA 98248
www.syndel.comRev: March 31, 2017
NDC Number: 50378-012-01
- Syndel USA Ovaprim Indexed Box v. 032320.jpg Syndel USA Ovaprim Indexed Label Sheet v. 032320.jpg
-
INGREDIENTS AND APPEARANCE
OVAPRIM
sgnrha and domperidone injection, solutionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:50378-012 Route of Administration INTRAPERITONEAL, INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALMON GONADOTROPIN RELEASING HORMONE D-ARG6 ANALOG ETHYL AMIDE ACETATE (UNII: 66KW0E903I) (SALMON GONADOTROPIN RELEASING HORMONE D-ARG6 ANALOG ETHYL AMIDE - UNII:WHI058YG4Q) SALMON GONADOTROPIN RELEASING HORMONE D-ARG6 ANALOG ETHYL AMIDE 20 ug in 1 mL Domperidone (UNII: 5587267Z69) (Domperidone - UNII:5587267Z69) Domperidone 10 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50378-012-01 10 mL in 1 VIAL, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Legally Marketed Unapproved New Animal Drugs for Minor Species MIF900001 02/11/2009 Labeler - Western Chemical Inc. (085803500) Registrant - Western Chemical Inc. (085803500) Establishment Name Address ID/FEI Business Operations Western Chemical Inc. 085803500 label, analysis