Label: ARCALYST- rilonacept injection, powder, lyophilized, for solution
- NDC Code(s): 73604-914-01, 73604-914-04
- Packager: Kiniksa Pharmaceuticals (UK), Ltd.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated March 7, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ARCALYST safely and effectively. See full prescribing information for ARCALYST. ARCALYST® (rilonacept) for injection, for ...These highlights do not include all the information needed to use ARCALYST safely and effectively. See full prescribing information for ARCALYST.
ARCALYST® (rilonacept) for injection, for subcutaneous use
Initial U.S. Approval: 2008RECENT MAJOR CHANGES
INDICATIONS AND USAGE
ARCALYST (rilonacept) is an interleukin-1 blocker indicated for:
- Treatment of Cryopyrin-Associated Periodic Syndromes (CAPS), including Familial Cold Autoinflammatory Syndrome (FCAS), and Muckle-Wells Syndrome (MWS) in adults and children 12 years and older (1.1, 14.1)
- Maintenance of remission of Deficiency of Interleukin-1 Receptor Antagonist (DIRA) in adults and pediatric patients weighing 10 kg or more (1.2, 14.2)
- Treatment of recurrent pericarditis (RP) and reduction in risk of recurrence in adults and children 12 years and older (1.3, 14.3)
DOSAGE AND ADMINISTRATION
Administer by subcutaneous injection (2.1)
-
CAPS, FCAS, MWS, and RP (2.2):
Adults:- –
- Loading dose: 320 mg, delivered as two 160 mg (2 mL) injections.
- –
- Maintenance dose: 160 mg (2 mL) injection once weekly.
- –
- Loading dose: 4.4 mg/kg, up to a maximum of 320 mg, delivered as 1 or 2 injections (not to exceed 2 mL/injection).
- –
- Maintenance dose: 2.2 mg/kg, up to a maximum of 160 mg (2 mL) injection, once weekly.
-
DIRA (2.3):
Adults and pediatric patients weighing 10 kg or more:- –
- 4.4 mg/kg up to a maximum of 320 mg, delivered as 1 or 2 injections (2 mL/injection) once weekly.
DOSAGE FORMS AND STRENGTHS
For injection: 220 mg of lyophilized powder in a single-dose vial for reconstitution. (3)
CONTRAINDICATIONS
None. (4)
WARNINGS AND PRECAUTIONS
- Serious Infections: Serious, life-threatening infections have been reported in patients taking ARCALYST. Do not initiate treatment with ARCALYST in patients with active or chronic infections. Discontinue treatment if a patient develops a serious infection. (5.1)
- Hypersensitivity Reactions: If a hypersensitivity reaction occurs, discontinue administration of ARCALYST and initiate appropriate therapy. (5.3)
- Immunizations: Avoid live vaccines. Update recommended vaccinations prior to initiation of therapy per current guidelines. (5.5)
ADVERSE REACTIONS
The most common adverse reactions reported by patients with CAPS and RP treated with ARCALYST are injection-site reactions and upper respiratory tract infections. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Kiniksa at 1-833-KINIKSA (1-833-546-4572) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2021
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Cryopyrin-Associated Periodic Syndromes, Familial Cold Autoinflammatory Syndrome and Muckle-Wells Syndrome
1.2 Deficiency of IL-1 Receptor Antagonist
1.3 Recurrent Pericarditis
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
2.2 Cryopyrin-Associated Periodic Syndromes, Familial Cold Autoinflammatory Syndrome, Muckle-Wells Syndrome and Recurrent Pericarditis
2.3 Deficiency of IL-1 Receptor Antagonist
2.4 Preparation for Administration
2.5 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Infections
5.2 Risk of Malignancy
5.3 Hypersensitivity Reactions
5.4 Lipid Profile Changes
5.5 Immunizations
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
6.2 Immunogenicity
7 DRUG INTERACTIONS
7.1 TNF-Blocking Agent and IL-1 Blocking Agent
7.2 Cytochrome P450 Substrates
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Patients with Renal Impairment
8.7 Patients with Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Cryopyrin-Associated Periodic Syndromes, Familial Cold Autoinflammatory Syndrome and Muckle-Wells Syndrome
14.2 Deficiency of IL-1 Receptor Antagonist
14.3 Recurrent Pericarditis
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE1.1 Cryopyrin-Associated Periodic Syndromes, Familial Cold Autoinflammatory Syndrome and Muckle-Wells Syndrome - ARCALYST® (rilonacept) is an interleukin-1 blocker indicated for the treatment of ...
1.1 Cryopyrin-Associated Periodic Syndromes, Familial Cold Autoinflammatory Syndrome and Muckle-Wells Syndrome
ARCALYST® (rilonacept) is an interleukin-1 blocker indicated for the treatment of Cryopyrin-Associated Periodic Syndromes (CAPS), including Familial Cold Autoinflammatory Syndrome (FCAS), and Muckle-Wells Syndrome (MWS) in adults and pediatric patients 12 years and older.
1.2 Deficiency of IL-1 Receptor Antagonist
ARCALYST is indicated for the maintenance of remission of Deficiency of Interleukin-1 Receptor Antagonist (DIRA) in adults and pediatric patients weighing at least 10 kg.
Close1.3 Recurrent Pericarditis
ARCALYST is indicated for the treatment of recurrent pericarditis (RP) and reduction in risk of recurrence in adults and pediatric patients 12 years and older.
-
2 DOSAGE AND ADMINISTRATION2.1 General Dosing Information - ARCALYST is for subcutaneous use only. 2.2 Cryopyrin-Associated Periodic Syndromes, Familial Cold Autoinflammatory Syndrome, Muckle-Wells Syndrome and ...
2.2 Cryopyrin-Associated Periodic Syndromes, Familial Cold Autoinflammatory Syndrome, Muckle-Wells Syndrome and Recurrent Pericarditis
Adults: Initiate treatment with a loading dose of 320 mg delivered as two, 2-mL subcutaneous injections of 160 mg each, administered on the same day at two different injection sites. Continue dosing with a once-weekly injection of 160 mg administered as a single, 2-mL subcutaneous injection.
Pediatric patients 12 years to 17 years: Initiate treatment with a loading dose of 4.4 mg/kg, up to a maximum dose of 320 mg, administered as one or two subcutaneous injections, not to exceed single-injection volume of 2 mL per injection site. If the initial dose is given as two injections, administer on the same day at two different sites. Continue dosing with a once-weekly injection of 2.2 mg/kg, up to a maximum of 160 mg, administered as a single subcutaneous injection, up to 2 mL.
If a once-weekly dose is missed, instruct the patient to administer the injection within 7 days from the missed dose and then resume the patient's original schedule. If the missed dose is not administered within 7 days, instruct the patient to administer the dose, starting a new schedule based on this date.
2.3 Deficiency of IL-1 Receptor Antagonist
Adults: The recommended dose of ARCALYST is 320 mg, once weekly, administered as two subcutaneous injections on the same day at two different sites with a maximum single-injection volume of 2 mL. ARCALYST should not be given more often than once weekly.
Pediatric patients weighing 10 kg or more: The recommended dose of ARCALYST is 4.4 mg/kg (up to a maximum of 320 mg), once weekly, administered as one or two subcutaneous injections with a maximum single-injection volume of 2 mL. If the dose is given as two injections, administer both on the same day, each one at a different site.
When switching from another IL-1 blocker, discontinue the IL-1 blocker and begin ARCALYST treatment at the time of the next dose [see Drug Interactions (7.1)].
2.4 Preparation for Administration
Reconstitute each single-dose vial of ARCALYST with 2.3 mL of preservative-free Sterile Water for Injection, USP (supplied separately) prior to subcutaneous administration of the drug.
Close2.5 Administration
Using aseptic technique, withdraw 2.3 mL of preservative-free Sterile Water for Injection through an 18-gauge, 1- or 1½-inch needle attached to a 3-mL syringe and inject the preservative-free Sterile Water for Injection, USP, into the drug product vial for reconstitution. The needle and syringe used for reconstitution with preservative-free Sterile Water for Injection, USP, should then be discarded and should not be used for subcutaneous injections. After the addition of preservative-free Sterile Water for Injection, USP, reconstitute the vial contents by shaking the vial for approximately one minute and then allowing it to sit for one minute. The resulting 80mg/mL solution is sufficient to allow a withdrawal volume of up to 2 mL for subcutaneous administration. The reconstituted solution is viscous, clear, colorless to pale yellow, and free from particulates. Prior to injection, inspect the reconstituted solution for any discoloration or particulate matter. Discard the solution if either is observed.
Using aseptic technique, withdraw the recommended dose volume, up to 2 mL (160 mg), of the solution with a new 18-gauge, 1- or 1½-inch needle attached to a new 3-mL syringe. For the subcutaneous injection, replace the needle with a new 26-gauge, ½-inch needle. EACH VIAL SHOULD BE USED FOR A SINGLE DOSE ONLY. Discard the vial after withdrawal of drug.
After reconstitution, ARCALYST may be kept at room temperature, but keep it protected from light, and use the solution within three hours after reconstitution. Discard unused portions of ARCALYST.
Rotate the sites for subcutaneous injection, such as the abdomen, thigh, or upper arm. Injections should never be administered at sites that are bruised, red, tender, or hard.
-
3 DOSAGE FORMS AND STRENGTHSFor injection: 220 mg of rilonacept as a white to off-white lyophilized powder for reconstitution in single-dose vials.
For injection: 220 mg of rilonacept as a white to off-white lyophilized powder for reconstitution in single-dose vials.
Close -
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Serious Infections - Interleukin-1 (IL-1) blockade may interfere with the immune response to infections. Treatment with another medication that works through inhibition of IL-1 has been ...
5.1 Serious Infections
Interleukin-1 (IL-1) blockade may interfere with the immune response to infections. Treatment with another medication that works through inhibition of IL-1 has been associated with an increased risk of serious infections, and serious infections have been reported in patients taking ARCALYST [see Clinical Studies (14)]. There was a greater incidence of infections in CAPS and RP patients on ARCALYST compared with placebo.
In the controlled portion of the CAPS study [see Clinical Studies (14.1)], severe infection (bronchitis) was reported in one patient receiving ARCALYST. In an open-label extension study in CAPS, one patient developed bacterial meningitis and died [see Adverse Reactions (6.1)].
In clinical studies, ARCALYST has not been administered concomitantly with tumor necrosis factor (TNF) inhibitors. An increased incidence of serious infections has been associated with administration of an IL-1 blocker in combination with TNF inhibitors. ARCALYST is not recommended for use with TNF inhibitors because this may increase the risk of serious infections.
Drugs that affect the immune system by blocking TNF have been associated with an increased risk of reactivation of latent tuberculosis (TB). It is possible that taking drugs such as ARCALYST that block IL-1 increases the risk of TB or other atypical or opportunistic infections. Refer to current practice guidelines for evaluation and treatment of possible latent tuberculosis infections before initiating therapy with ARCALYST.
Treatment with ARCALYST should not be initiated in patients with an active or chronic infection. Discontinue ARCALYST if a patient develops a serious infection.
5.2 Risk of Malignancy
The impact of treatment with ARCALYST on the development of malignancies is not known. Treatment with immunosuppressants, including ARCALYST, may result in an increase in the risk of malignancies.
5.3 Hypersensitivity Reactions
Hypersensitivity reactions associated with ARCALYST occurred in clinical trials. If a hypersensitivity reaction occurs, discontinue ARCALYST and initiate appropriate therapy.
5.4 Lipid Profile Changes
Cholesterol and lipid levels may be reduced in patients with chronic inflammation. Increases in non-fasting lipid profile parameters occurred in patients treated with ARCALYST in clinical trials. Monitor patients' lipid profiles and consider lipid-lowering therapies if needed, based on cardiovascular risk factors and current guidelines [see Adverse Reactions (6.1)].
Close5.5 Immunizations
Since no data are available on the risks of secondary transmission of infection by live vaccines in patients receiving ARCALYST, avoid administration of live vaccines during treatment with ARCALYST.
No data are available on the effectiveness of vaccines in patients receiving ARCALYST. Since ARCALYST may interfere with normal immune response to new antigens, vaccines may not be effective in patients receiving ARCALYST.
Because IL-1 blockade may interfere with immune response to infections, it is recommended that prior to initiation of therapy with ARCALYST, adult and pediatric patients receive all recommended vaccinations, as per current immunization guidelines, including pneumococcal vaccine and inactivated influenza vaccine.
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling. Serious Infections [see Warnings and Precautions (5.1)] Risk of Malignancy[see Warnings and ...
The following clinically significant adverse reactions are described elsewhere in the labeling.
- Serious Infections [see Warnings and Precautions (5.1)]
- Risk of Malignancy[see Warnings and Precautions (5.2)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- Lipid Profile Changes [see Warnings and Precautions (5.4)]
6.1 Clinical Trial Experience
Clinical trials are conducted under widely varying conditions and, as such, adverse reaction rates observed cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The data described herein reflect exposure to ARCALYST in over 2,000 patients who received at least one dose, including approximately 1700 exposed to 160 mg or more, of whom 151 patients were exposed for at least 6 months and 111 patients for at least one year. These included patients with CAPS and RP, patients with other diseases, and healthy volunteers.
CAPS
Approximately 60 patients with CAPS were treated weekly with 160 mg of ARCALYST. The pivotal trial population included 47 patients with CAPS. These patients were between the ages of 22 and 78 years (average 51 years). Thirty-one patients were female and 16 were male. All of the patients were White/Caucasian. Six pediatric patients (12 to17 years) were enrolled directly into the open-label extension phase of the trial.
Part A of the clinical trial was conducted in patients with CAPS who were naïve to treatment with ARCALYST. Part A of the study was a randomized, double-blind, placebo-controlled, six-week study comparing ARCALYST to placebo [see Clinical Studies (14)]. Table 1 reflects the frequency of adverse events reported by at least two patients during Part A.
Table 1: Most Frequent Adverse Reactions in Patients with CAPS (Part A, Reported by at Least Two Patients) Adverse Event ARCALYST
160 mg
(n = 23)Placebo
(n= 24)Any AE 17 (74%) 13 (54%) Injection-site reactions 11 (48%) 3 (13%) Upper respiratory tract infection 6 (26%) 1 (4%) Nausea 1 (4%) 3 (13%) Diarrhea 1 (4%) 3 (13%) Sinusitis 2 (9%) 1 (4%) Abdominal pain upper 0 2 (8%) Cough 2 (9%) 0 Hypoesthesia 2 (9%) 0 Stomach discomfort 1 (4%) 1 (4%) Urinary tract infection 1 (4%) 1 (4%) DIRA
In a 2-year, open-label study, 6 pediatric patients with DIRA, 3 years to 6 years of age, received a 2.2 to 4.4 mg/kg dose of ARCALYST once weekly [see Clinical Studies (14.2)]. The safety profile was generally consistent with that seen in patients with CAPS. The most common adverse reactions were upper respiratory infection (6 of 6), rash (5 of 6), otitis media (3 of 6), pharyngitis (3 of 6) and rhinorrhea (3 of 6).
RP
In the RP phase 3 study, a total of 86 patients received at least one dose of ARCALYST with a median treatment duration of 9 months [see Clinical Studies (14.3)]. Of the patients, 49 (57%) were female and 37 (43%) were male; 93% were White/Caucasian. The mean age was 44.7 years. Seven patients (8%) were aged 12-17 years old. No new adverse reactions were identified in this study.
Adverse Reactions of Special Interest
Injection-Site Reactions
In patients with CAPS or RP, the most common and consistently reported adverse event associated with ARCALYST was injection-site reaction (ISR). The ISRs included erythema, swelling, pruritus, mass, bruising, inflammation, pain, edema, dermatitis, discomfort, urticaria, vesicles, warmth and hemorrhage. Most injection-site reactions lasted for one to two days.
Infections
During Part A in the CAPS study, the incidence of patients reporting infections was greater with ARCALYST (48%) than with placebo (17%). In Part B, randomized withdrawal, the incidence of infections was similar in the ARCALYST (18%) and the placebo patients (22%). Part A of the trial was initiated in the winter months, while Part B was predominantly performed in the summer months.
In placebo-controlled studies across a variety of patient populations encompassing 360 patients treated with rilonacept and 179 treated with placebo, the incidence of infections was 34% and 27% (2.15 per patient-exposure year and 1.81 per patient-exposure year), respectively, for rilonacept and placebo.
Serious Infections
Six serious infections were reported by four patients during the CAPS clinical program: Mycobacterium intracellulare infection; gastrointestinal bleeding and colitis; sinusitis and bronchitis; and Streptococcus pneumoniae meningitis [see Adverse Reactions (6)].
One patient receiving ARCALYST for an unapproved indication in another study developed an infection in his olecranon bursa with Mycobacterium intracellulare. The patient was on chronic glucocorticoid treatment. The infection occurred after an intraarticular glucocorticoid injection into the bursa with subsequent local exposure to a suspected source of mycobacteria. The patient recovered after the administration of the appropriate antimicrobial therapy. One patient treated for another unapproved indication developed bronchitis/sinusitis, which resulted in hospitalization. One patient died in an open-label study of CAPS from Streptococcus pneumoniae meningitis.
Changes in Hematologic Parameters Laboratory Changes
One patient in a study in an unapproved indication developed transient neutropenia (ANC < 1 × 109/L) after receiving a large dose (2000 mg intravenously) of ARCALYST. The patient did not experience any infection associated with the neutropenia.
Lipid Profile Changes
Patients with CAPS treated with ARCALYST experienced increases in their mean total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides. The mean increases from baseline for total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides were 19 mg/dL, 2 mg/dL, 10 mg/dL, and 57 mg/dL, respectively, after 6 weeks of open-label therapy.
Close6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. The data reflect the percentage of patients whose test results were positive for antibodies to the rilonacept receptor domains in specific assays. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay is highly dependent on several factors including assay sensitivity and specificity, assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to rilonacept with the incidence of antibodies in other studies or to other products may be misleading.
Antibodies directed against the receptor domains of rilonacept were detected by an ELISA assay in patients with CAPS after treatment with ARCALYST. Nineteen of 55 patients (35%) who had received ARCALYST for at least 6 weeks tested positive for treatment-emergent binding antibodies on at least one occasion. Of the 19, seven tested positive at the last assessment (Week 18 or 24 of the open-label extension period), and five patients tested positive for neutralizing antibodies on at least one occasion. There was no correlation of antibody activity and either clinical effectiveness or safety.
In the Phase 3 study of patients with RP, there were no patients who tested positive for antibodies at baseline. At any point in time, 26 out of 86 (30%) subjects tested positive at any assessment and of these, 6 tested positive for neutralizing antibodies (NAb). At the last assessment, 10 subjects remained positive for anti-drug antibodies (ADA) and 1 subject remained positive for NAb. There was no correlation of antibody activity and either clinical effectiveness or safety.
-
7 DRUG INTERACTIONS7.1 TNF-Blocking Agent and IL-1 Blocking Agent - Specific drug interaction studies have not been conducted with ARCALYST. Concomitant administration of another drug that blocks IL-1 with a ...
7.1 TNF-Blocking Agent and IL-1 Blocking Agent
Specific drug interaction studies have not been conducted with ARCALYST. Concomitant administration of another drug that blocks IL-1 with a TNF-blocking agent in another patient population has been associated with an increased risk of serious infections and an increased risk of neutropenia. The concomitant administration of ARCALYST with TNF-blocking agents may also result in similar toxicities and is not recommended [see Warnings and Precautions (5.1)]. The concomitant administration of ARCALYST with other drugs that block IL-1 has not been studied. Based upon the potential for pharmacologic interactions between rilonacept and a recombinant IL-1ra, concomitant administration of ARCALYST and other agents that block IL-1 or its receptors is not recommended.
Close7.2 Cytochrome P450 Substrates
The formation of CYP450 enzymes is suppressed by increased levels of cytokines (e.g., IL-1) during chronic inflammation. Thus it is expected that for a molecule that binds to IL-1, such as rilonacept, the formation of CYP450 enzymes could be normalized. This is clinically relevant for CYP450 substrates with a narrow therapeutic index, where the dose is individually adjusted (e.g., warfarin). Upon initiation of ARCALYST in patients being treated with these types of medicinal products, therapeutic monitoring of the effect or drug concentration should be performed, and the individual dose of the medicinal product may need to be adjusted.
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Rare pregnancy outcomes reported postmarketing and from clinical trials, with very limited use of ARCALYST in pregnant women, are insufficient to evaluate for a ...
8.1 Pregnancy
Risk Summary
Rare pregnancy outcomes reported postmarketing and from clinical trials, with very limited use of ARCALYST in pregnant women, are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. There may be risks to the mother and fetus associated with Cryopyrin Associated Periodic Syndromes (CAPS) (see Clinical Considerations). In an animal reproduction study, subcutaneous administration of rilonacept to pregnant monkeys during the period of organogenesis was complicated by losses of drug exposure as the study progressed, due to anti-drug antibody formation at all doses, but a dose-related increase in exposure was still evident. There were no treatment-related effects on fetal survival or development of malformations with doses up to 11 times the maximum recommended human dose (MRHD). Increased incidences of lumbar ribs, a skeletal variation, were observed in fetuses at doses approximately 2 times the MRHD and higher that slightly exceeded incidences in both control animals and the historical control database (see Data). There were findings of multiple fusion and absence of the ribs and thoracic vertebral bodies and arches in one fetus of the only pregnant monkey with exposure to rilonacept during the later period of gestation associated with a dose approximately 6 times the MRHD (see Data). The relationship of these findings in a single fetus to drug treatment was unclear, as these findings were not evident in fetuses from pregnant monkeys that had higher exposures to rilonacept during the period of organogenesis associated with a dose approximately 11 times the MRHD.
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies is 2%–4% and of miscarriage is 15%–20%, respectively.
Data
Animal Data
In an embryo-fetal development study, pregnant cynomolgus monkeys received rilonacept at subcutaneous doses of 0, 5, 15 or 30 mg/kg given twice a week from gestation days 20 to 48. The study was complicated by losses of drug exposure as the study progressed, due to anti-drug antibody formation at all doses, but a dose-related increase in exposure was still evident. There were no treatment-related effects on fetal survival or development of malformations with doses up to 11 times the MRHD (on a mg/kg basis with maternal subcutaneous doses up to 30 mg/kg). Increased incidences of lumbar ribs, a skeletal variation, were observed in fetuses at doses approximately 2 times the MRHD and higher (on a mg/kg basis with maternal subcutaneous doses of 5 mg/kg and higher) that slightly exceeded incidences in both control animals and the historical control database. There were findings of multiple fusion and absence of the ribs and thoracic vertebral bodies and arches in one fetus of the only pregnant monkey with exposure to rilonacept during the later period of gestation associated with a dose 6 times the MRHD (on a mg/kg basis with a maternal subcutaneous dose of 15 mg/kg). The relationship of these findings in a single fetus to drug treatment was unclear, as these findings were not evident in fetuses from pregnant monkeys that had higher exposures to rilonacept during the period of organogenesis associated with a dose approximately 11 times the MRHD (on a mg/kg basis with a maternal subcutaneous dose of 30 mg/kg). All doses of rilonacept reduced maternal serum levels of estradiol up to 64% compared to controls. In pre- and postnatal development studies in the mouse model using a murine analog of rilonacept (subcutaneous doses of 0, 20, 100 or 200 mg/kg), there was a small increase in the number of stillbirths in dams treated with 200 mg/kg three times per week.
8.2 Lactation
Risk Summary
There is no information on the presence of rilonacept in either human or animal milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ARCALYST and any potential adverse effects on the breastfed child from ARCALYST or from the underlying maternal condition.
8.4 Pediatric Use
Cryopyrin-Associated Periodic Syndromes (CAPS) and Recurrent Pericarditis (RP)
Safety and effectiveness in pediatric patients with CAPS and RP below the age of 12 years have not been established.
Six pediatric patients with CAPS between the ages of 12 and 16 were treated with ARCALYST at a weekly subcutaneous dose of 2.2 mg/kg (up to a maximum of 160 mg) for 24 weeks during the open-label extension phase. These patients showed improvement from baseline in their symptom scores and in objective markers of inflammation (e.g., Serum Amyloid A and C-Reactive Protein). The adverse reactions included injection-site reactions and upper respiratory symptoms as were commonly seen in adult patients.
The trough drug levels for four pediatric patients measured at the end of the weekly dose interval (mean 20 mcg/mL, range 3.6 to 33 mcg/mL) were similar to those observed in adult patients with CAPS (mean 24 mcg/mL, range 7 to 56 mcg/mL).
In the Phase 3 study RHAPSODY, 7 patients aged 12 years to 17 years were treated with ARCALYST subcutaneously with an initial loading dose of 4.4 mg/kg (up to a maximum of 320 mg) followed by 2.2 mg/kg (up to a maximum of 160 mg) weekly. These patients were treated for a median time of 15 weeks. There were no apparent differences in efficacy, safety or tolerability across age groups.
When administered to pregnant primates, rilonacept treatment may have contributed to alterations in bone ossification in the fetus. It is not known if ARCALYST will alter bone development in pediatric patients. Pediatric patients treated with ARCALYST should undergo appropriate monitoring for growth and development. [see Use in Specific Populations (8.1)]
Deficiency of Interleukin-1 Receptor Antagonist (DIRA)
Safety and effectiveness in pediatric patients with DIRA weighing 10 kg or more have been established [see Adverse Reactions (6.1) and Clinical Studies (14.2)]. Safety and effectiveness of ARCALYST have not been established in pediatric patients weighing less than 10 kg for maintenance of remission of DIRA.
8.5 Geriatric Use
In the placebo-controlled clinical studies, 78 patients randomized to treatment with ARCALYST were ≥ 65 years of age, and 6 were ≥ 75 years of age. In the CAPS clinical trial, efficacy, safety and tolerability were generally similar in elderly patients as compared to younger adults; however, only ten patients ≥ 65 years old participated in the trial. In an open-label extension study of CAPS, a 71-year-old woman developed bacterial meningitis and died [see Adverse Reactions (6.1)]. Age did not appear to have a significant effect on steady-state trough concentrations in the clinical study.
8.6 Patients with Renal Impairment
No studies have been conducted to evaluate the pharmacokinetics of rilonacept administered subcutaneously in patients with renal impairment.
Close8.7 Patients with Hepatic Impairment
No studies have been conducted to evaluate the pharmacokinetics of rilonacept administered subcutaneously in patients with hepatic impairment.
-
10 OVERDOSAGEThere have been no reports of overdose with ARCALYST. Maximum weekly doses of up to 320 mg have been administered subcutaneously for up to approximately 18 months in a small number of patients ...
There have been no reports of overdose with ARCALYST. Maximum weekly doses of up to 320 mg have been administered subcutaneously for up to approximately 18 months in a small number of patients with CAPS and up to 6 months in patients with an unapproved indication in clinical trials without evidence of dose-limiting toxicities. In addition, ARCALYST given intravenously at doses up to 2000 mg monthly in another patient population for up to six months was tolerated without dose-limiting toxicities. The maximum amount of ARCALYST that can be safely administered has not been determined.
In case of overdose, it is recommended that the patient be monitored for any signs or symptoms of adverse reactions or effects, and appropriate symptomatic treatment instituted immediately.
Close -
11 DESCRIPTIONRilonacept is a dimeric fusion protein consisting of the ligand-binding domains of the extracellular portions of the human interleukin-1 receptor component (IL-1R1) and IL-1 receptor accessory ...
Rilonacept is a dimeric fusion protein consisting of the ligand-binding domains of the extracellular portions of the human interleukin-1 receptor component (IL-1R1) and IL-1 receptor accessory protein (IL-1RAcP) linked in-line to the Fc portion of human IgG1. Rilonacept has a molecular weight of approximately 251 kDa. Rilonacept is expressed in recombinant Chinese hamster ovary (CHO) cells.
ARCALYST is supplied in single-dose vials containing a sterile, white to off-white lyophilized powder. Each vial of ARCALYST is to be reconstituted with 2.3 mL of Sterile Water for Injection, USP. A volume of up to 2 mL can be withdrawn, which is designed to deliver 160 mg for subcutaneous administration only. The resulting solution is viscous, clear, colorless to pale yellow, and essentially free from particulates. Each vial contains 220 mg rilonacept. After reconstitution, each vial contains rilonacept (80 mg/mL), glycine (10 mg/mL), histidine (46 mM), L-arginine (50 mM), polyethylene glycol 3350 (30 mg/mL), and sucrose (20 mg/mL) at a pH of 6.5. No preservatives are present.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Rilonacept is an interleukin-1 alpha (IL-1α) and interleukin-1 beta (IL-1β) cytokine trap. Rilonacept blocks IL-1 signaling by acting as a soluble decoy receptor that ...
12.1 Mechanism of Action
Rilonacept is an interleukin-1 alpha (IL-1α) and interleukin-1 beta (IL-1β) cytokine trap. Rilonacept blocks IL-1 signaling by acting as a soluble decoy receptor that binds both IL-1α and IL-1β and prevents its interaction with cell surface receptors. Rilonacept also binds interleukin-1 receptor antagonist (IL-1ra). The equilibrium dissociation constants for rilonacept binding to IL-1α, IL-1β, and IL-1ra were 1.4 pM, 0.5 pM, and 6.1 pM, respectively.
CAPS refers to rare genetic syndromes generally caused by mutations in the NLRP-3 [nucleotide-binding domain, leucine rich family (NLR), pyrin domain containing 3] gene (also known as Cold-Induced Autoinflammatory Syndrome-1 [CIAS1]). CAPS disorders are inherited in an autosomal dominant pattern with male and female offspring equally affected. Features common to all disorders include fever, urticaria-like rash, arthralgia, myalgia, fatigue, and conjunctivitis.
In most cases, inflammation in CAPS is associated with mutations in the NLRP-3 gene, which encodes the protein cryopyrin, an important component of the inflammasome. Cryopyrin regulates the protease caspase-1 and controls the activation of interleukin-1 beta (IL-1β). Mutations in NLRP-3 result in an overactive inflammasome, resulting in excessive release of activated IL-1β that drives inflammation.
DIRA is an autoinflammatory, autosomal recessive disorder caused by loss of function mutations in the IL1RN gene, which encodes IL-1 receptor antagonist (IL-1ra), resulting in unopposed signaling of the proinflammatory cytokines IL-1α and IL-1β through the IL-1 receptor.
Interleukin-1 (IL-1) is a key cytokine that mediates the pathophysiology of many inflammatory processes, and it has been implicated as a causative factor in pericarditis. IL-1α and IL-1β bind to the universally expressed cell surface receptor, IL-1 Receptor type-1, triggering a cascade of inflammatory mediators. Pre-formed IL-1α is released by damaged/inflamed pericardial cells and may contribute to the maintenance and amplification of inflammation via activation of the NLRP3 inflammasome, which then augments the inflammatory response by production of IL-1β in a cascade amplification system.
12.2 Pharmacodynamics
C-Reactive Protein (CRP) and Serum Amyloid A (SAA) are indicators of inflammatory disease activity that are elevated in patients with CAPS. Elevated SAA has been associated with the development of systemic amyloidosis in patients with CAPS. Compared to placebo, treatment with ARCALYST resulted in sustained reductions from baseline in mean serum CRP and SAA to normal levels during the clinical trial. ARCALYST also normalized mean SAA from elevated levels.
CRP is also an indicator of inflammation in DIRA. Maintenance of CRP reduction was observed in a clinical trial of pediatric patients with DIRA [see Clinical Studies (14.2)].
CRP is also a recognized indicator of inflammation in pericarditis. Treatment with rilonacept was observed in a clinical trial with RP to be associated with resolution of acute pericarditis episodes, including rapid and sustained reduction in CRP, with a median time to normalization of 7 days [see Clinical Studies (14.3)].
Close12.3 Pharmacokinetics
The average trough levels of rilonacept were approximately 24 mcg/mL at steady state following weekly subcutaneous doses of 160 mg for up to 48 weeks in patients with CAPS. The steady state appeared to be reached by 6 weeks.
The average trough levels of rilonacept were approximately 23 mcg/mL at steady state, and the circulation half-life in vivo was approximately 7 days following a 320 mg loading dose and weekly subcutaneous doses of 160 mg for up to 36 weeks in patients with RP. Steady state appeared to be reached by approximately 2 weeks. No pharmacokinetic data are available in patients with hepatic or renal impairment.
No specific study was conducted to evaluate the effect of age, gender, or body weight on rilonacept exposure. Based on limited data obtained from the Phase 3 study RHAPSODY, steady state trough concentrations were similar between male and female patients. Age (26 to78 years) and body weight (50 to120 kg) did not appear to have a meaningful effect on trough rilonacept concentrations. The effect of race has not been assessed because of low numbers of non-Caucasian patients in the CAPS and RP programs, reflecting the epidemiology of these diseases.
In pediatric patients with DIRA (3 to 6 years of age and body weight 12.7 to 19.9 kg) who received weekly subcutaneous doses of 4.4 mg/kg of ARCALYST, the average steady-state trough levels ranged from 63.5 to 74.0 mg/mL during the period of 6 to 24 months from the start of treatment with ARCALYST.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term animal studies have not been performed to evaluate the carcinogenic potential of rilonacept. A murine analog of rilonacept ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential of rilonacept.
A murine analog of rilonacept had no effects on fertility and reproductive performance in male and female mice at subcutaneous doses up to 200 mg/kg three times per week.
-
14 CLINICAL STUDIES14.1 Cryopyrin-Associated Periodic Syndromes, Familial Cold Autoinflammatory Syndrome and Muckle-Wells Syndrome - The safety and efficacy of ARCALYST for the treatment of CAPS was demonstrated ...
14.1 Cryopyrin-Associated Periodic Syndromes, Familial Cold Autoinflammatory Syndrome and Muckle-Wells Syndrome
The safety and efficacy of ARCALYST for the treatment of CAPS was demonstrated in a randomized, double-blind, placebo-controlled study (NCT00288704) with two parts (A and B) conducted sequentially in the same patients with FCAS and MWS.
Part A was a 6-week, randomized, double-blind, parallel-group period comparing ARCALYST at a dose of 160 mg weekly after an initial loading dose of 320 mg to placebo. Part B followed immediately after Part A and consisted of a 9-week, patient-blind period during which all patients received ARCALYST 160 mg weekly, followed by a 9-week, double-blind, randomized withdrawal period in which patients were randomly assigned to either remain on ARCALYST 160 mg weekly or to receive placebo. Patients were then given the option to enroll in a 24-week, open-label treatment extension phase in which all patients were treated with ARCALYST 160 mg weekly.
Using a daily diary questionnaire, patients rated the following five signs and symptoms of CAPS: joint pain, rash, feeling of fever/chills, eye redness/pain, and fatigue, each on a scale of 0 (none, no severity) to 10 (very severe). The study evaluated the mean symptom score using the change from baseline to the end of treatment.
The changes in mean symptom scores for the randomized parallel-group period (Part A) and the randomized withdrawal period (Part B) of the study are shown in Table 2. ARCALYST-treated patients had a larger reduction in the mean symptom score in Part A compared to placebo-treated patients. In Part B, mean symptom scores increased more in patients withdrawn to placebo compared to patients who remained on ARCALYST.
Table 2: Mean Symptom Scores Part A Placebo
(n=24)ARCALYST
(n=23)Part B Placebo
(n=23)ARCALYST
(n=22)Pre-treatment Baseline Period
(Weeks -3 to 0)2.4 3.1 Active ARCALYST Baseline Period
(Weeks 13 to 15)0.2 0.3 Endpoint Period
(Weeks 4 to 6)2.1 0.5 Endpoint Period
(Weeks 22 to 24)1.2 0.4 LS* Mean Change from Baseline to Endpoint -0.5 -2.4 LS* Mean Change from Baseline to Endpoint 0.9 0.1 95% confidence interval for difference between treatment groups (-2.4, -1.3)† 95% confidence interval for difference between treatment groups (-1.3, -0.4)† Daily mean symptom scores over time for Part A are shown in Figure 1.
Figure 1: Group Mean Daily Symptom Scores by Treatment Group in Part A and Single-blind ARCALYST Treatment Phase from Week -3 to Week 15
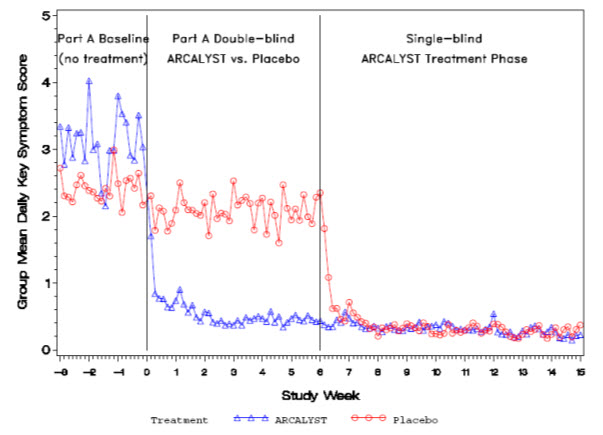
Improvement in symptom scores was noted within several days of initiation of ARCALYST therapy in most patients.
In Part A, patients treated with ARCALYST experienced more improvement in each of the five components of the composite endpoint (joint pain, rash, feeling of fever/chills, eye redness/pain, and fatigue) than placebo-treated patients.
In Part A, a higher proportion of patients in the ARCALYST group experienced improvement from baseline in the composite score by at least 30% (96% vs. 29% of patients), by at least 50% (87% vs. 8%) and by at least 75% (70% vs. 0%) compared to the placebo group.
Serum Amyloid A (SAA) and C-Reactive Protein (CRP) levels are acute phase reactants that are typically elevated in patients with CAPS with active disease. During Part A, mean levels of CRP decreased versus baseline for the ARCALYST treated patients, while there was no change for those on placebo (Table 3). ARCALYST also led to a decrease in SAA versus baseline to levels within the normal range.
Table 3. Mean Serum Amyloid A and C-Reactive Protein Levels Over Time in Part A Part A ARCALYST Placebo SAA
(normal range: 0.7 – 6.4 mg/L)(n=22) (n=24) Pre-treatment Baseline 60 110 Week 6 4 110 CRP
(normal range: 0.0 – 8.4 mg/L)(n= 21) (n=24) Pre-treatment Baseline 22 30 Week 6 2 28 During the open-label extension, reductions in mean symptom scores, serum CRP, and serum SAA levels were maintained for up to one year.
14.2 Deficiency of IL-1 Receptor Antagonist
The safety and efficacy of ARCALYST for the maintenance of remission of DIRA were demonstrated in a 2-year, open-label study (NCT01801449) of 6 pediatric patients who previously experienced clinical benefit from daily injections of an IL-1 receptor antagonist, anakinra. The study population included patients with a loss-of-function IL1RN mutations. Patients had a median age at baseline of 4.8 years (range 3.3 to 6.2), and stopped anakinra treatment 24 hours before initiation of ARCALYST.
Remission was defined using the following criteria: diary score of < 0.5 (reflecting no fever, skin rash and bone pain), acute phase reactants (<0.5 mg/dL CRP), absence of objective skin rash, and no radiological evidence of active bone lesions.
Following an ARCALYST loading dose of 4.4 mg/kg subcutaneously, patients received a once-weekly maintenance dose of 2.2 mg/kg (up to a maximum 160 mg), and were assessed for remission and possible dose escalation. During the first 3 months of ARCALYST administration at the 2.2 mg/kg dose, five of 6 patients exhibited recurrence of pustular rash and therefore the dose was escalated to 4.4 mg/kg once weekly (up to a maximum of 320 mg). One patient remained on the 2.2 mg/kg once-weekly dose.
All patients met the primary endpoint of the study, remission at 6 months and sustained the remission for the remainder of the 2-year study. No patient required steroid use during the study.
Close14.3 Recurrent Pericarditis
The efficacy and safety of ARCALYST were evaluated in the Phase 3 study RHAPSODY (NCT03737110), a double-blind, placebo-controlled, randomized withdrawal, multinational study. The study consisted of a 12-week run-in followed by a double-blind, placebo-controlled, randomized withdrawal period.
In the run-in period, adult patients received a loading dose of ARCALYST 320 mg followed by 160 mg weekly. Patients between 12 and 17 years of age received a loading dose of ARCALYST 4.4 mg/kg (up to 320 mg) followed by 2.2 mg/kg (up to 160 mg) weekly. During the run-in period, patients tapered and discontinued standard-of-care therapies.
In the withdrawal period, patients were randomized 1:1 to remain on ARCALYST 160 mg weekly or to receive placebo. The randomized withdrawal period continued until the pre-specified number of primary efficacy endpoint events (pericarditis recurrence) had accrued.
Patients recorded scores for pericarditis pain in a daily diary using the 0 to 10 NRS scale. Measurements of CRP, electrocardiograms, and echocardiograms were conducted at intervals during study visits and to assess pericarditis recurrence
Patients who experienced pericarditis recurrence were eligible for open-label ARCALYST (bailout).
A total of 86 patients (mean age 45 years [range 13-78], 57% females) with symptomatic pericarditis recurrence were enrolled and received study treatment. Of these, 73 (85%) had a diagnosis of "idiopathic" pericarditis, and the remainder post-cardiac injury pericarditis. The mean duration of disease was 2.4 years with a mean of 4.4 pericarditis events per year including the qualifying pericarditis event (0-10 point Numerical Rating Scale [NRS] ≥ 4 and CRP ≥ 1 mg/dL). Mean qualifying NRS pain score was 6.2, and mean qualifying CRP level was 6.2 mg/dL.
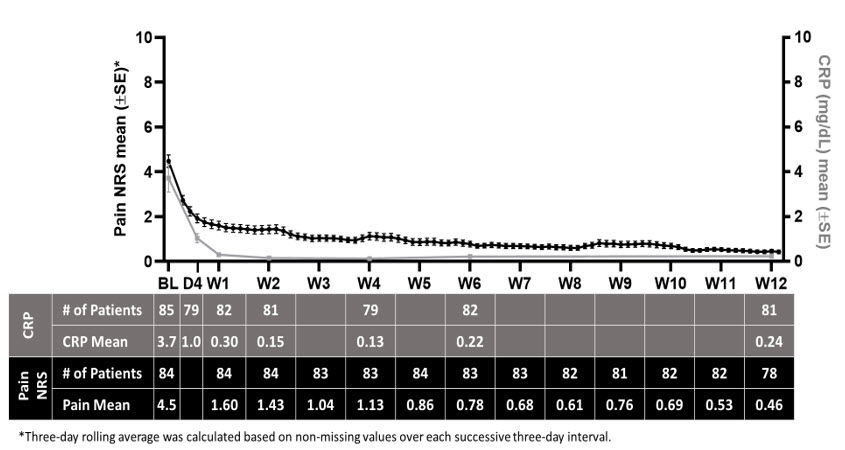
During the run-in period, daily NRS pain scores and CRP levels decreased as shown in Figure 2.
Figure 2: Summary of Pain NRS and CRP Means
Time to treatment response (NRS ≤ 2 and CRP ≤ 0.5 mg/dL) is shown in Table 4. The median time to treatment response was 5.0 days. All patients were required to taper off standard-of-care pericarditis medications before randomization, and median time to rilonacept monotherapy was 7.9 weeks during the run-in period.
Of the 86 patients enrolled, 41 (48%) were on treatment with corticosteroids (CS) at baseline (mean treatment duration of 20 weeks).
Table 4. Time to Treatment Response: Run-in Period ARCALYST
(N=86)Subjects with baseline NRS score >2 or CRP > 0.5 mg/dL 79 Subjects achieving treatment response 77 (97%) Days to treatment response (median; 95% CI) 5 (4, 7) Time to monotherapy (median; 95% CI) 8 (7, 8) weeks The primary efficacy endpoint was time to first adjudicated pericarditis recurrence (based on pain, CRP and clinical signs) in the event-driven withdrawal period. Of 61 patients randomized, 23 patients (74%) in the placebo arm had a recurrence compared with 2 patients (7%) in the rilonacept arm who temporarily discontinued treatment for 1 – 3 doses. The median time-to-recurrence on rilonacept could not be estimated because too few events occurred and was 8.6 weeks (95% CI 4.0, 11.7) on placebo with a hazard ratio of 0.04 (p < 0.0001); rilonacept reduced the risk of recurrence by 96% (Figure 3).
The two recurrence events in the rilonacept group happened in association with temporary interruptions of the trial-drug regimen, of one to three weekly doses. In the placebo group, all 23 patients who had pericarditis recurrence received bailout rilonacept, with resolution of the episodes.
Figure 3. Primary Efficacy Endpoint for RHAPSODY
Kaplan-Meier Curves for Time to Pericarditis Recurrence Based on ITT Analysis Set
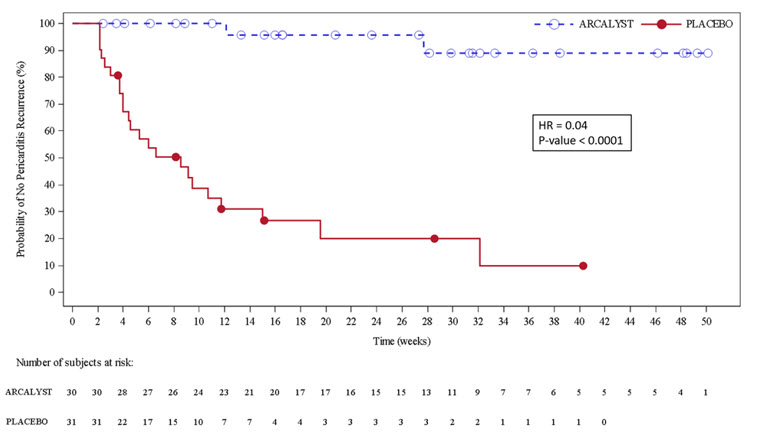
Secondary efficacy endpoints were the proportion of patients with maintenance of clinical response, the percentage of trial days with none/minimal pericarditis pain (NRS ≤ 2) each measured at Week 16 of the withdrawal period. Results are summarized in Table 5.
Table 5. Secondary Efficacy Endpoints for RHAPSODY ARCALYST
n=21Placebo
n=20Increase
(%)p-value Number of patients who maintained response at Week 16 17 4 61 0.0002 Percentage of days with NRS ≤2 92 40 52 <0.0001 -
16 HOW SUPPLIED/STORAGE AND HANDLINGARCALYST (rilonacept) for injection is supplied as a sterile, white to off-white, preservative-free, lyophilized powder in single-dose vials. Each 220 mg vial of ARCALYST is supplied in a carton ...
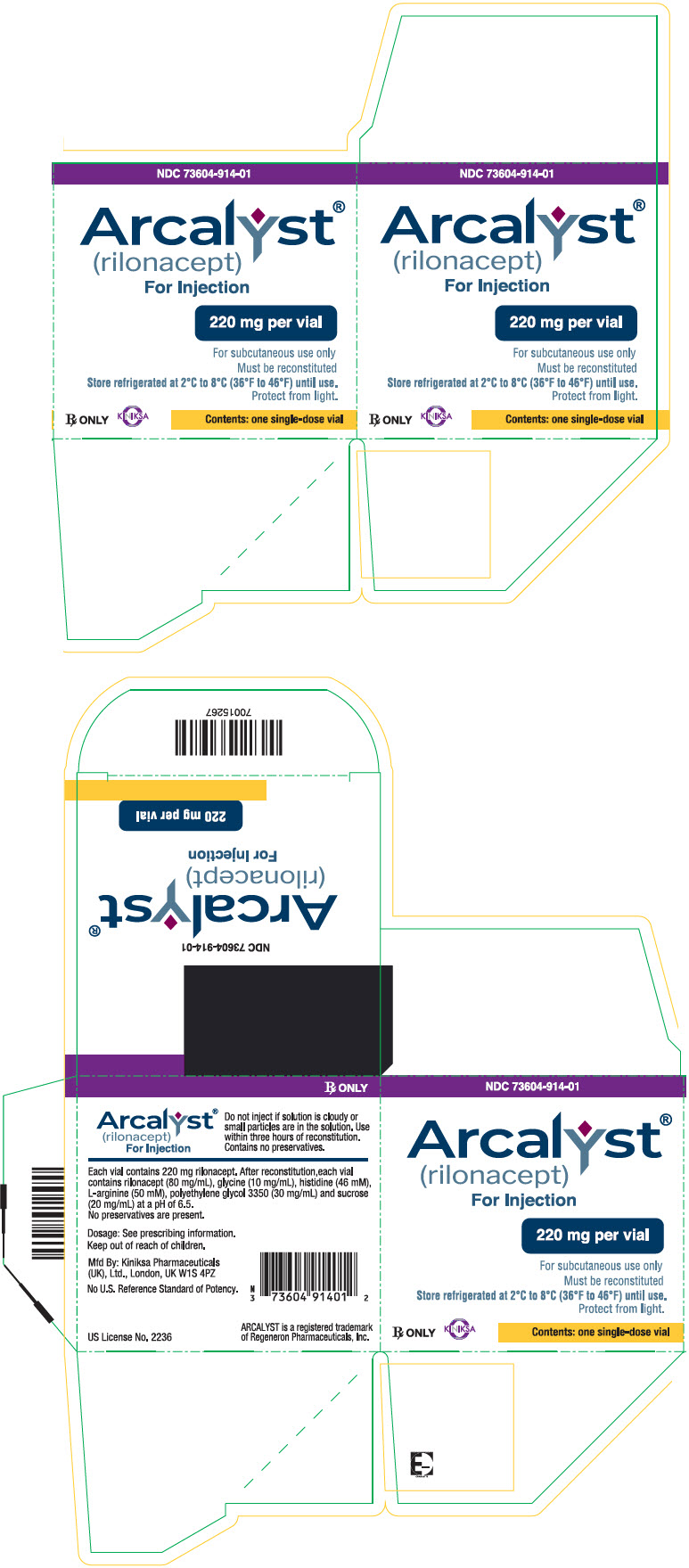
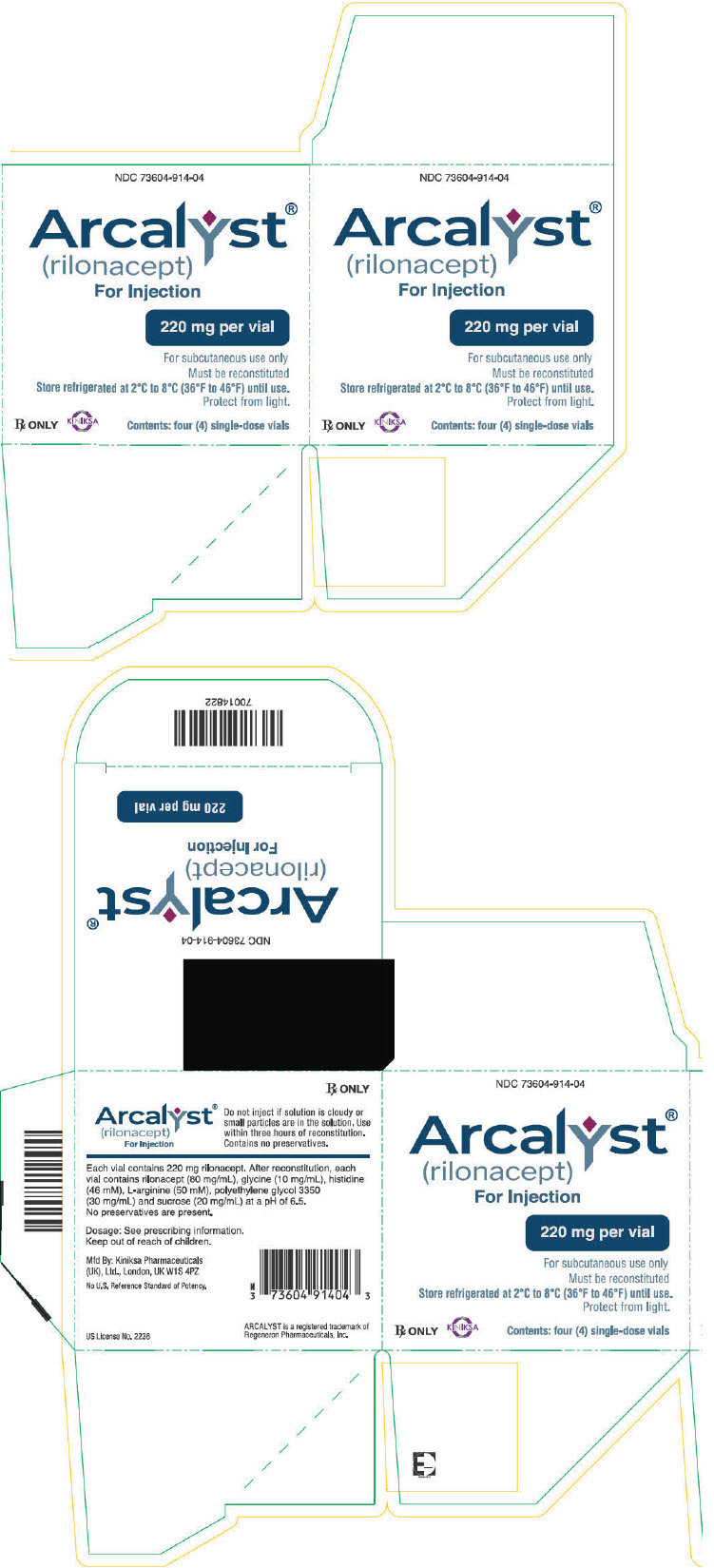
ARCALYST (rilonacept) for injection is supplied as a sterile, white to off-white, preservative-free, lyophilized powder in single-dose vials.
Each 220 mg vial of ARCALYST is supplied in a carton containing one vial (NDC 73604-914-01) or four vials (NDC 73604-914-04).
CloseStore refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light. Do not use beyond the date stamped on the label. After reconstitution, ARCALYST may be kept at room temperature, protected from light [see Dosage and Administration (2.5)].
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Assessment of Patient's or Caregiver's Administration of ARCALYST: The first ...
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Assessment of Patient's or Caregiver's Administration of ARCALYST: The first injection of ARCALYST should be performed under the supervision of a qualified healthcare professional. If a patient or caregiver is to administer ARCALYST, instruct them on aseptic reconstitution of the lyophilized product and injection technique. The ability to inject subcutaneously should be assessed to ensure proper administration of ARCALYST, including rotation of injection sites. (See Patient Information Leaflet for ARCALYST®). ARCALYST should be reconstituted with preservative-free Sterile Water for Injection to be provided by the pharmacy. If the total volume of dose needed is greater than 2 mL, provide instruction about how to divide the total dose and how to administer the 2 injections. Remind patients to discard vials with unused product. A sharps disposal container should be used for disposal of vials, needles and syringes. Instruct patients or caregivers in proper vial, syringe, and needle disposal, and they should be cautioned against reuse of these items [see Dosage and Administration (2.5)].
Injection-site Reactions: Explain to patients that almost half of the patients in the clinical trials experienced a reaction at the injection site. Injection-site reactions may include pain, erythema, swelling, pruritus, bruising, mass, inflammation, dermatitis, edema, urticaria, vesicles, warmth, and hemorrhage. Caution patients to avoid injecting into an area that is already swollen or red. Any persistent reaction should be brought to the attention of the prescribing physician [see Adverse Reactions (6.1)].
Infections: Caution patients that ARCALYST has been associated with serious, life-threatening infections, and not to initiate treatment with ARCALYST if they have a chronic or active infection. Advise patients to contact their healthcare professional immediately if they develop an infection after starting ARCALYST. Treatment with ARCALYST should be discontinued if a patient develops a serious infection. Advise patients not to take any IL-1 blocking drug, including ARCALYST, if they are also taking a drug that blocks TNF such as etanercept, infliximab, or adalimumab. Use of ARCALYST with other IL-1 blocking agents, such as anakinra, is not recommended [see Warnings and Precautions (5.1)].
CloseVaccinations: Prior to initiation of therapy with ARCALYST, review the vaccination history with adult and pediatric patients, parent(s), and/or caregiver relative to current medical guidelines for vaccine use, including taking into account the potential of increased risk of infection during treatment with ARCALYST [see Warnings and Precautions (5.5)].
-
SPL UNCLASSIFIED SECTIONManufactured by: Kiniksa Pharmaceuticals (UK), Ltd. London, UK W1S 4PZ - U.S. License Number 2236 - 1-833-KINIKSA (1-833-546-4572) NDC 73604-914-04 and NDC 73604-914-01 - ARCALYST® is a registered ...
Manufactured by:
Kiniksa Pharmaceuticals (UK), Ltd.
London, UK W1S 4PZ
U.S. License Number 22361-833-KINIKSA (1-833-546-4572)
NDC 73604-914-04 and NDC 73604-914-01ARCALYST® is a registered trademark of Regeneron Pharmaceuticals, Inc.
© 2021, Kiniksa Pharmaceuticals (UK), Ltd.All rights reserved.
Close -
PATIENT PACKAGE INSERTPatient Information - ARCALYST® (ARK-a-list) (rilonacept) For Injection - For Subcutaneous Use - Read the Patient Information that comes with ARCALYST before you or your child start taking ...
Patient Information
ARCALYST® (ARK-a-list)
(rilonacept)
For Injection
For Subcutaneous UseRead the Patient Information that comes with ARCALYST before you or your child start taking it and each time you or your child get a refill. There may be new information. This Patient Information leaflet does not take the place of talking with the healthcare provider about your or your child's medical condition or treatment. What is the most important information I should know about ARCALYST?
ARCALYST can affect your or your child's immune system. ARCALYST can lower the ability of your or your child's immune system to fight infections. Serious infections, including life-threatening infections and death, have happened in people taking ARCALYST. Taking ARCALYST can make you or your child more likely to get infections, including life-threatening serious infections, or may make any infection that you or your child have worse.
You or your child should not begin treatment with ARCALYST if you or your child have an infection or have infections that keep coming back (chronic infection).
After starting ARCALYST, if you or your child get an infection, any sign of an infection, including a fever, cough, flu-like symptoms, or have any open sores on your body, call the healthcare provider right away. Treatment with ARCALYST should be stopped if you or your child get a serious infection.
You or your child should not take medicines that block tumor necrosis factor (TNF), such as Enbrel® (etanercept), Humira® (adalimumab), or Remicade® (infliximab), while you or your child are taking ARCALYST. You or your child should also not take other medicines that block interleukin-1 (IL-1), such as Kineret® (anakinra), while taking ARCALYST. Taking ARCALYST with any of these medicines may increase your or your child's risk of getting a serious infection.
Before starting treatment with ARCALYST, tell the healthcare provider if you or your child:- think you have an infection.
- are being treated for an infection.
- have signs of an infection, such as fever, cough, or flu-like symptoms.
- have any open sores on your body.
- have a history of infections that keep coming back.
- have asthma. People with asthma may have an increased risk of infection.
- have diabetes or an immune system problem. People with these problems have a higher chance for infections.
- have tuberculosis (TB), or if you have been in close contact with someone who has had tuberculosis.
- have or have had HIV, hepatitis B, or hepatitis C.
- take other medicines that affect your immune system.
What is ARCALYST? - ARCALYST is a prescription medicine called an interleukin-1 (IL-1) blocker.
- ARCALYST is used to treat adults and children 12 years and older with Cryopyrin-Associated Periodic Syndromes (CAPS), including Familial Cold Autoinflammatory Syndrome (FCAS), and Muckle-Wells Syndrome (MWS)
- ARCALYST is used to maintain control of symptoms of Deficiency of Interleukin-1 Receptor Antagonist (DIRA) in adults and children weighing at least 22 pounds (10 kg).
- ARCALYST is used to treat Recurrent Pericarditis (RP) and reduce the risk of recurrence in adults and children 12 years and older.
It is not known if ARCALYST is safe and effective in children with DIRA weighing less than 22 pounds (10 kg).What should I tell my or my child's healthcare provider before taking ARCALYST?
ARCALYST may not be right for you or your child. Before taking ARCALYST, tell your or your child's healthcare provider about all medical conditions, including if you or your child:- are scheduled to receive any vaccines. You or your child should not receive live vaccines if you take ARCALYST. See "What is the most important information I should know about ARCALYST?"
- are pregnant or plan to become pregnant. It is not known if ARCALYST will harm your unborn child. Tell the healthcare provider right away if you become pregnant while taking ARCALYST.
- are breastfeeding or plan to breastfeed. It is not known if ARCALYST passes into breast milk. Talk to the healthcare provider about the best way to feed your or your child's baby during treatment with ARCALYST.
Especially tell the healthcare provider if you or your child take other medicines that affect the immune system, such as:- See "What is the most important information I should know about ARCALYST?"
- Corticosteroids
If you have any questions about any of this information, ask the healthcare provider.How should I take ARCALYST?
See the Instructions for Use at the end of this Patient Information leaflet.- Take or give ARCALYST exactly as prescribed by the healthcare provider.
- ARCALYST is given by injection under the skin (subcutaneous injection) 1 time each week.
- ARCALYST should not be given more than 1 time each week.
- The healthcare provider will tell you how much ARCALYST to take or give your child, and show you how to prepare and give the injection.
- Do not try to give ARCALYST injections until you are sure that you understand how to prepare and inject your dose. Call the healthcare provider or pharmacist if you have any questions, or if you would like more training.
- If you or your child take too much ARCALYST, call the healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of ARCALYST?
ARCALYST can cause serious side effects, including:- See "What is the most important information I should know about ARCALYST?"
- Risk of Cancer. Medicines that affect the immune system may increase the risk of getting cancer.
- Allergic Reaction. Stop taking or giving ARCALYST and call the healthcare provider or get emergency care right away if you or your child get any of the following symptoms of an allergic reaction while taking ARCALYST:
- rash
- swollen face
- trouble breathing
- Changes in your blood cholesterol and triglycerides (lipids). Your or your child's healthcare provider will do blood tests to check for these changes.
- Injection-site reactions including: pain, redness, swelling, itching, bruising, lumps, inflammation, skin rash, blisters, warmth, and bleeding at the injection site.
- Upper respiratory tract infections
- Joint and muscle aches in RP
- Upper respiratory infection
- Rash
- Ear infection
- Sore throat
- Runny nose
How should I store ARCALYST? - Keep ARCALYST in the carton it comes in to protect from light.
- Store ARCALYST in the refrigerator from 36°F to 46°F (2°C to 8°C). Call your pharmacy if you have any questions.
- Refrigerated ARCALYST can be used until the expiration date printed on the vial and carton.
- ARCALYST may be kept at room temperature after mixing with Sterile Water for Injection, USP, and should be used within 3 hours of mixing. Keep ARCALYST away from light.
General Information about the safe and effective use of ARCALYST.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ARCALYST for a condition for which it was not prescribed. Do not give ARCALYST to other people even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about ARCALYST that is written for health professionals.What are the ingredients in ARCALYST?
Active ingredient: rilonacept.
Inactive ingredients: glycine, histidine, L-arginine, polyethylene glycol 3350 and sucrose.Manufactured by:
Close
Kiniksa Pharmaceuticals (UK), Ltd.
London, UK W1S 4PZ
U.S. License Number 2236 -
Instructions for UseARCALYST® (ARK-a-list) (rilonacept) For Injection - For Subcutaneous Use - It is important that you read, understand and follow these instructions before you or your child start using ARCALYST so ...
ARCALYST® (ARK-a-list)
(rilonacept)
For Injection
For Subcutaneous UseIt is important that you read, understand and follow these instructions before you or your child start using ARCALYST so that you prepare and inject the medicine the right way.
Do not try to inject ARCALYST until you have been shown the right way to give the injections by your or your child's healthcare provider.
How do I prepare and give an ARCALYST injection?
Step 1: Setting up for an injection
- Choose a table or other flat area to set up the supplies for your injection. Be sure the area is clean or clean it with an antiseptic or soap and water.
- Wash your hands well with soap and water about 20 seconds, and dry with a clean towel.
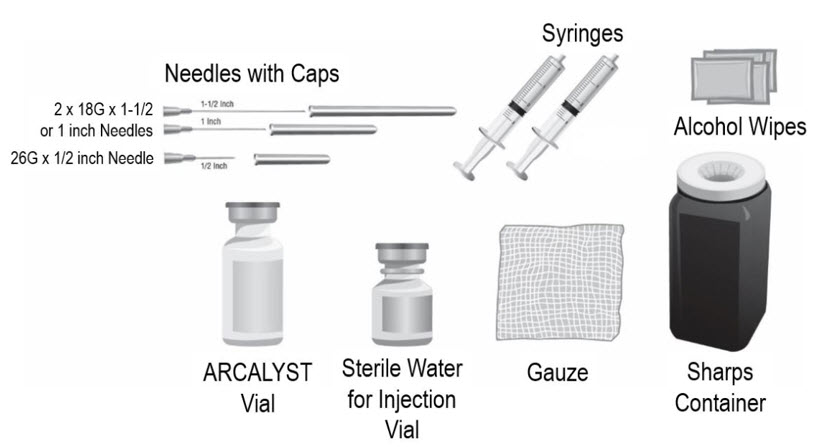
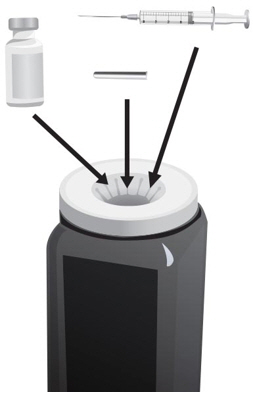
- Put the following supplies on the cleaned area for each injection (see Figure A):
Supplies needed to give your ARCALYST injection:
- 1 vial of ARCALYST (powder for mixing)
Additional supplies needed (available from the pharmacy):
- 1 vial of preservative-free Sterile Water for Injection, USP.
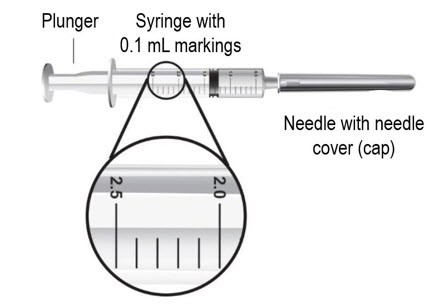
- 2 sterile, 3-milliliter (mL) disposable syringes with markings at each 0.1 mL (see Figure B):
- 1 syringe for mixing ARCALYST
- 1 syringe for injection
- 2 sterile disposable needles (18-gauge, 1-inch or 1½-inch) and 1 sterile disposable needle (26-gauge, ½-inch) with needle covers
- 1 18-gauge needle for transferring the Sterile Water for Injection, USP, to the rilonacept vial
- 1 18-gauge needle for withdrawing the mixed solution
- 1 26-gauge needle for injection
- 4 alcohol wipes
- 1 gauze pad
- 1 sharps disposal container for throwing away (disposing of) used needles, syringes, and vials
Note:
- Do not use Sterile Water for Injection, USP, syringes or needles other than those provided by your pharmacy. Contact your pharmacy if you need replacement syringes or needles.
- Do not touch the needles or the rubber stoppers on the vials with your hands. If you touch the rubber stopper, clean it with a new alcohol wipe.
- If you touch a needle or the needle touches any surface, throw away the entire syringe in the sharps disposal container and use a new syringe.
- Do not reuse needles or syringes.
- To protect yourself and others from possible needlesticks, it is very important to throw away each syringe, with the needle attached, in the sharps disposal container right after use.
Step 2: Preparing the vials
- 1.
- Check the expiration date on the carton of ARCALYST. Do not use the vial if the expiration date has passed. Contact your pharmacy if the expiration date has passed.
- 2.
- Check the expiration date on the vial of Sterile Water for Injection, USP. Do not use the vial if the expiration date has passed. Contact your pharmacy for assistance.
- 3.
- Remove the protective plastic caps from both vials.
- 4.
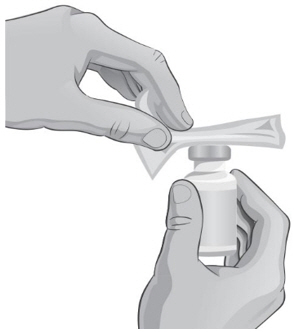
- Clean the top of each vial with an alcohol wipe. Use 1 wipe for each vial and wipe in 1 direction around the top of the vial (see Figure C).
- 5.
- Check the expiration date on the needle. Do not use the needle if the expiration date has passed. Contact your pharmacy if the expiration date has passed.
- 6.
- Open the wrapper that contains 1 of the 18-gauge needles by pulling apart the tabs and set it aside for later use. Do not remove the needle cover. This needle will be used to mix the water with ARCALYST powder in the vial.
- 7.
- Check the expiration date on the syringe. Do not use the syringe if the expiration date has passed. Contact your pharmacy if the expiration date has passed.
- 8.
- Open the wrapper that contains the syringe by pulling apart the tabs (see Figure D).
- 9.
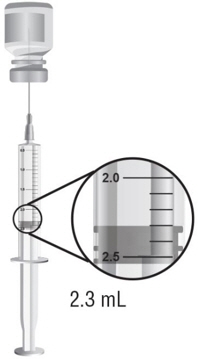
- Hold the barrel of the syringe with one hand and use your other hand to twist the 18-gauge needle with the cover onto the tip of the syringe until it fits firmly (see Figure E).
- 10.
- Hold the syringe upright at eye level. With the needle cover still on the 18-gauge needle, pull back the plunger to the 2.3 mL mark to fill the syringe with air (see Figure F).
- 11.
- Hold the syringe in one hand and use the other hand to pull the needle cover straight off. Do not twist the needle as you pull off the cover. Place the needle cover aside. Hold the syringe in the hand that you will use to mix your medicine. Hold the Sterile Water for Injection, USP, vial on a firm surface with your other hand. Slowly insert the needle straight through the rubber stopper. Do not bend the needle. Push the plunger in all the way to push the air into the vial (see Figure G).
- 12.
- Hold the vial in one hand and the syringe in the other hand and carefully turn the vial upside down so that the needle is pointing straight up (see Figure H).
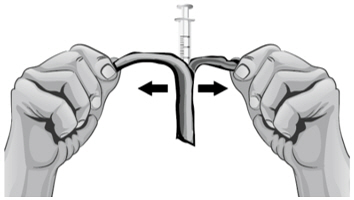
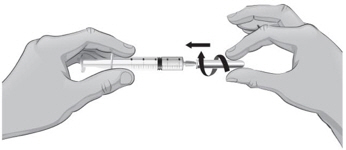
- 13.
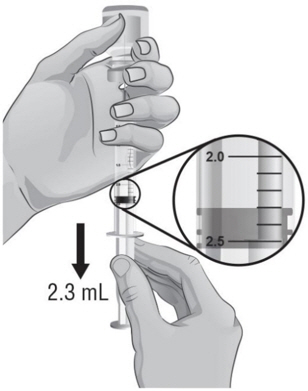
- Make sure the tip of the needle is covered by the liquid and slowly pull back on the plunger to the 2.3 mL mark to withdraw the Sterile Water for Injection, USP, from the vial (see Figure H).
- 14.
- Keep the vial upside down and gently tap the syringe with your fingers until any air bubbles rise to the top of the syringe.
- 15.
- To remove the air bubbles, gently push in the plunger so only the air is pushed out of the syringe and back into the bottle.
- 16.
- After removing the air bubbles, check the syringe to be sure that the right amount of Sterile Water for Injection, USP, has been drawn into the syringe (see Figure I).
- 17.
- Carefully remove the syringe with the 18-gauge needle from the Sterile Water for Injection, USP, vial. Do not touch the needle.
Step 3: Mixing ARCALYST
- 18.
- With one hand, hold the ARCALYST vial on a firm surface.
- 19.
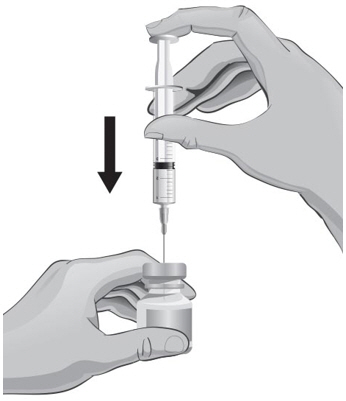
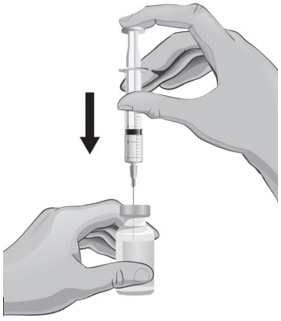
- With the other hand, take the syringe with the 18-gauge needle that contains the Sterile Water for Injection, USP, and slowly insert the needle straight down through the rubber stopper of the ARCALYST vial.
- 20.
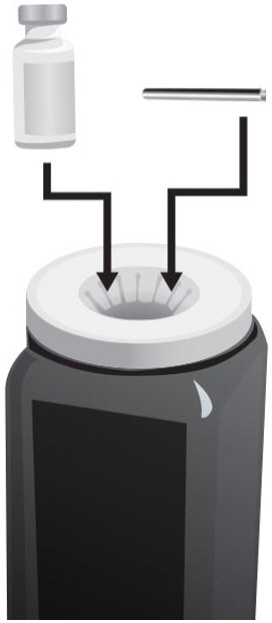
- Gently push the plunger in all the way to inject the Sterile Water for Injection, USP, into the vial, aiming the stream of Sterile Water for Injection, USP, down the side of the vial into the powder (see Figure J).
- 21.
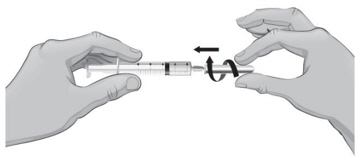
- Remove the syringe and needle from the rubber stopper and throw away the needle, syringe, and Sterile Water for Injection, USP, vial in the sharps disposal container. Do not try to put the needle cover back on the needle (see Figure K).
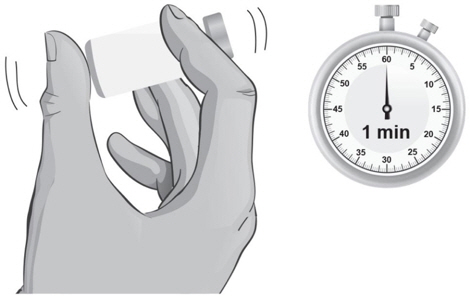
- 22.
- Hold the vial containing the ARCALYST and Sterile Water for Injection, USP, sideways (not upright) as shown (see Figure L). Do not touch the rubber stopper. Quickly shake the vial back and forth (side-to-side) for about 1 minute.
- 23.
- Put the vial back on the table and let the vial sit for about 1 minute.
- 24.
- Check the vial for any particles or clumps of powder that have not dissolved.
- 25.
- If the powder has not completely dissolved, shake the vial quickly back and forth for 30 seconds more. Let the vial sit for about 1 minute.
- 26.
- Repeat Step 25 until the powder is completely dissolved and the solution is clear (see Figure M).
- 27.
- The mixed ARCALYST should be thick, clear, and colorless to pale yellow. Do not use the mixed liquid if it is discolored or cloudy, or if particles are in it.
Note: Contact your pharmacy to report any mixed ARCALYST that is discolored, cloudy or contains particles. - 28.
- ARCALYST may be kept at room temperature after mixing. ARCALYST should be used within 3 hours of mixing. Keep ARCALYST away from light.
Step 4: Preparing the Injection
- 29.
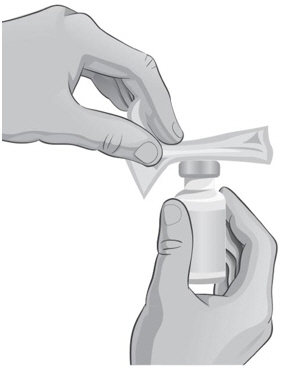
- Hold the ARCALYST vial in one hand and wipe in 1 direction around the top of the ARCALYST vial with a new alcohol wipe with the other hand (see Figure N).
- 30.
- Take a new sterile, disposable 18-gauge needle and attach it firmly to a new syringe without removing the needle cover (see Figures O and P).
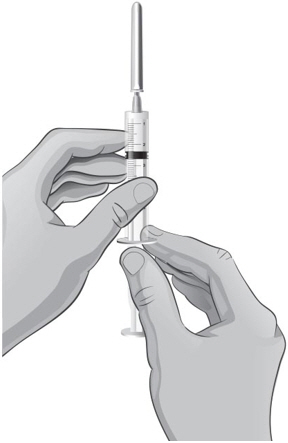
- 31.
- To draw air into the syringe, hold the syringe upright at eye level. Do not remove the needle cover. Pull back the plunger on the syringe to the mark that is equal to the amount of mixed ARCALYST that the healthcare provider has prescribed for you to inject (see Figure Q).
- 32.
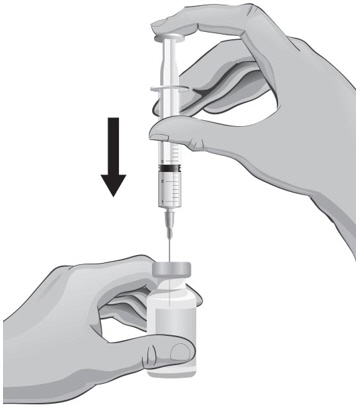
- Hold the syringe in one hand and use the other hand to pull the needle cover straight off. Do not twist the needle as you pull off the cover. Place the needle cover aside and be careful not to touch the needle. Keep the ARCALYST vial on a flat firm surface and slowly insert the needle straight down through the rubber stopper. Push the plunger down and inject all of the air into the vial (see Figure R).
- 33.
- Hold the vial in one hand and the syringe in the other hand and carefully turn the vial upside down so that the needle is pointing straight up. Hold the vial at eye level.
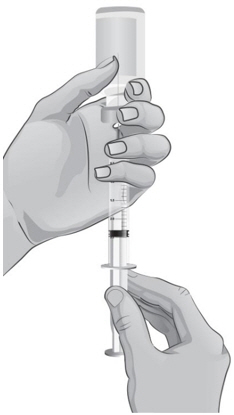
- 34.
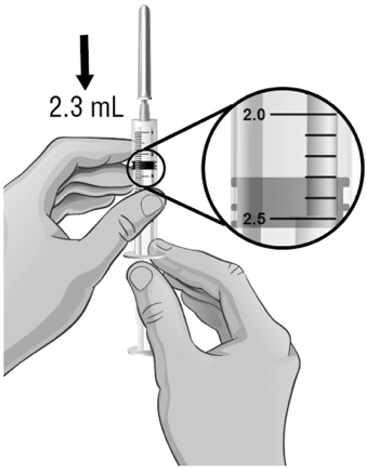
- Keep the tip of the needle in the liquid and slowly pull back on the plunger to the mark on the syringe that matches the amount of medicine prescribed by your or your child's healthcare provider (see Figure S).
Note: The maximum amount of medicine that you can withdraw from 1 vial of ARCALYST is 2 mL. If the amount of medicine you need to withdraw for your or your child's dose of ARCALYST is more than 2 mL, you will need to use 2 vials. The healthcare provider will tell you the right amount of medicine to withdraw from the 2 vials and how to give the 2 injections. Always use new syringes and needles for each injection. - 35.
- Keep the vial upside down with the needle straight up, and gently tap the syringe until any air bubbles rise to the top of the syringe (see Figure T).
It is important to remove air bubbles so that you withdraw the right amount of medicine from the vial.
- 36.
- To remove the air bubbles, slowly and gently push in the plunger so only the air is pushed through the needle.
- 37.
- Check to make sure that you have the amount of medicine prescribed by the healthcare provider in the syringe. Remove the syringe with the needle from the vial.
- 38.
- You will now prepare to switch needles.
- 39.
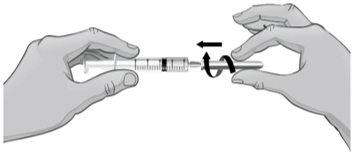
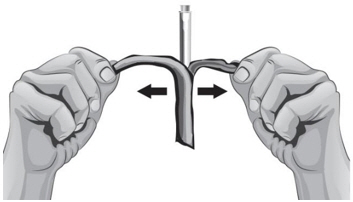
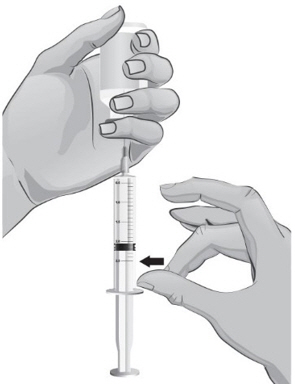
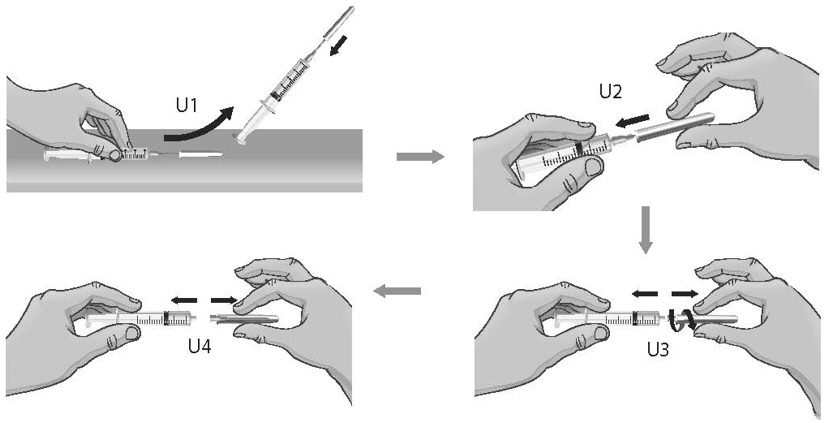
- To remove the 18-gauge needle and replace it with the new 26-gauge needle for injection, place the syringe with the 18-gauge needle and the needle cap on a flat surface (see Figure U1). Use one hand to slide the 18-gauge needle into the needle cap and scoop upwards to cover the needle (see Figure U2).
- 40.
- When the needle is covered, push the needle cap toward the syringe to fully attach it with one hand to prevent an accidental stick with the needle (see Figure U2). Twist off and remove the 18-gauge needle with the needle cap (see Figures U3 and U4).
- 41.
- Open a new sterile, disposable 26-gauge needle (see Figure V) and attach securely to the syringe without removing the needle cover (see Figure W).
- 42.
- Throw away the ARCALYST vial and 18-gauge needle that still has the needle cover attached to it in the sharps disposal container even if there is medicine left in the vial (see Figure X). Do not use any vial of ARCALYST more than 1 time.
Step 5: Giving the Injection
- 43.
- ARCALYST is given by injection into the tissue directly under the skin (subcutaneous injection). Do not inject ARCALYST into any muscle, vein, or artery.
You should change (rotate) the injection site each time you inject ARCALYST.
If you need to use 2 vials and give 2 injections for your or your child's prescribed dose of ARCALYST, you should use 2 different injection sites.
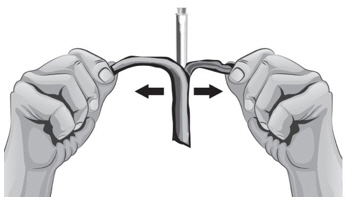
Changing injection sites helps to prevent irritation and allows the medicine to be completely absorbed. Ask your or your child's healthcare provider if you have any questions about changing injection sites.- Do not inject into skin that is tender, red, or hard. If an area is tender or feels hardened, choose another site for injection until the tenderness or hardening goes away.
- Tell your or your child's healthcare provider about any skin reactions including redness, swelling, or hardening of the skin.
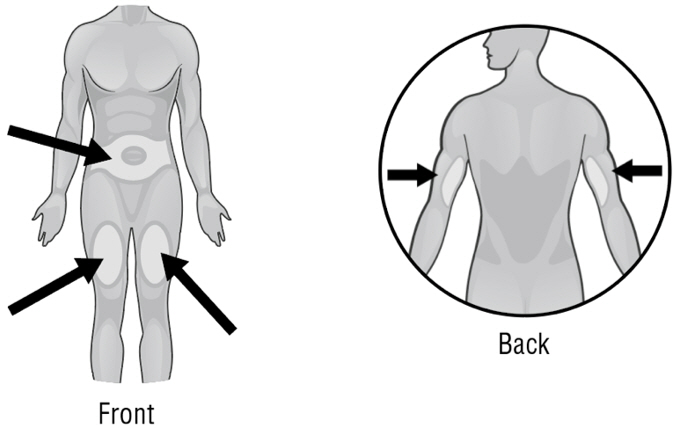
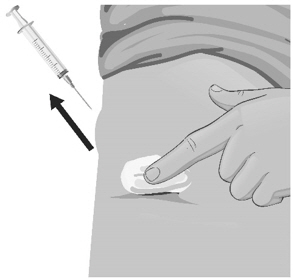
- Areas where you may inject ARCALYST include the left and right sides of the abdomen, and the left and right thighs. If you are giving the injection to your child or someone else, the upper left and right arms may also be used for injection (see Figure Y):
- 44.
- Choose the area for the injection. Clean the area in a circular motion with a new alcohol wipe. Begin at the center of the injection site and move outward. Let the alcohol air dry completely.
- 45.
- Remove the needle cover and hold the syringe in one hand like you would hold a pencil.
- 46.
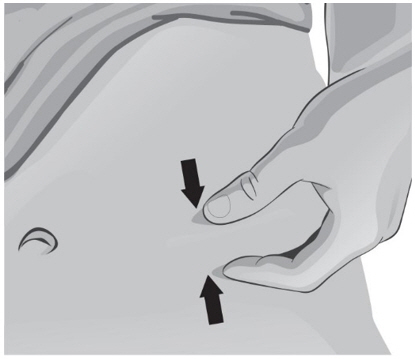
- With the other hand, gently pinch a fold of skin at the cleaned injection site (see Figure Z).
- 47.
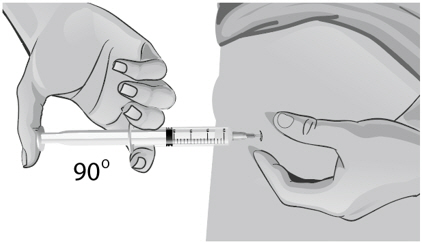
- Use a quick "dart like" motion to insert the needle straight into the skin at a 90-degree angle (see Figure AA). Do not push down on the plunger while inserting the needle into the skin.
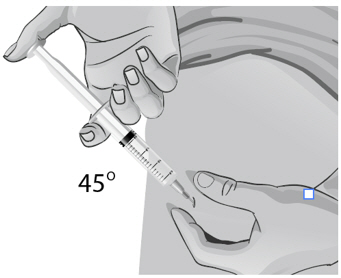
For small children or people with little fat under the skin, you may need to hold the syringe and needle at a 45-degree angle (see Figure BB).
- 48.
- After the needle is completely in the skin, let go of the pinched skin.
- 49.
- With your free hand, hold the syringe near the bottom. Gently pull back the plunger. If blood comes into the syringe, the needle has entered a blood vessel. Remove the needle and throw away (discard) the syringe and needle in the sharps disposal container. Start over with "Step 1: Setting up for an injection" using new supplies (syringes, needles, alcohol swabs, gauze pad, new vials of ARCALYST and Sterile Water for Injection, USP).
- 50.
- If no blood comes into the syringe, inject all the medicine in the syringe at a slow, steady rate, pushing the plunger all the way down. It may take up to 30 seconds to inject the entire dose.
- 51.
- Pull the needle out of the skin and hold a gauze pad over the injection site for several seconds (see Figure CC).
- 52.
- Do not replace the needle cover. Throw away the vials, used syringes and needles in an FDA-cleared sharps disposal container (see Figure DD). Do not throw away vials, needles, or syringes in the household trash or recycle.
If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- Do not reuse or share your syringes with other people.
- Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this.
- Do not recycle your used sharps disposal container.
- 53.
- Keep the sharps disposal container out of the reach of children.
- 54.
- Used alcohol wipes can be thrown away in the household trash.
Contact your or your child's healthcare provider right away with any questions or concerns about ARCALYST.
This Patient Information and Instructions for Use have been approved by the U.S. Food and Drug Administration.
ENBREL, HUMIRA, KINERET, and REMICADE are trademarks of Immunex Corporation, AbbVie Biotechnology Ltd., Sobi, Inc., and Janssen Biotech, Inc., respectively.
Manufactured by:
Kiniksa Pharmaceuticals (UK), Ltd.
London, UK W1S 4PZ
U.S. License Number 2236
NDC 73604-914-04NDC 73604-914-01
For more information about ARCALYST, call 1-833-KINIKSA (1-833-546-4572), or visit www.ARCALYST.com.
ARCALYST is a registered trademark of Regeneron Pharmaceuticals, Inc.
© 2021, Kiniksa Pharmaceuticals (UK), Ltd. All rights reserved.
Revised: 05/2021
Close -
PRINCIPAL DISPLAY PANEL - 220 mg Vial Carton - NDC 73604-914-04NDC 73604-914-04 - Arcalyst® (rilonacept) For Injection - 220 mg per vial - For subcutaneous use only - Must be reconstituted - Store refrigerated at 2°C to 8°C (36°F to 46°F) until use. Protect from ...
NDC 73604-914-04
Arcalyst®
(rilonacept)
For Injection220 mg per vial
For subcutaneous use only
Must be reconstituted
Store refrigerated at 2°C to 8°C (36°F to 46°F) until use.
Protect from light.Rx ONLY
KINIKSA
Contents: four (4) single-dose vialsClose
-
PRINCIPAL DISPLAY PANEL - 220 mg Vial Carton - NDC 73604-914-01NDC 73604-914-01 - Arcalyst® (rilonacept) For Injection - 220 mg per vial - For subcutaneous use only. Must be reconstituted. Store refrigerated at 2°C to 8°C (36°F to 46°F) until use. Protect from ...
NDC 73604-914-01
Arcalyst®
(rilonacept)
For Injection220 mg per vial
For subcutaneous use only.
Must be reconstituted.
Store refrigerated at 2°C to 8°C (36°F to 46°F) until use.
Protect from light.Rx ONLY
KINIKSA
Contents: one single-dose vialClose
-
INGREDIENTS AND APPEARANCEProduct Information
ARCALYST rilonacept injection, powder, lyophilized, for solution Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:73604-914 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RILONACEPT (UNII: 8K80YB5GMG) (RILONACEPT - UNII:8K80YB5GMG) RILONACEPT 160 mg in 2 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) ARGININE (UNII: 94ZLA3W45F) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) SUCROSE (UNII: C151H8M554) GLYCINE (UNII: TE7660XO1C) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73604-914-04 4 in 1 CARTON 03/24/2008 1 2 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 2 NDC:73604-914-01 1 in 1 CARTON 06/18/2021 2 2 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125249 02/27/2008 Labeler - Kiniksa Pharmaceuticals (UK), Ltd. (224568777) Registrant - Kiniksa Pharmaceuticals (UK), Ltd. (224568777) Establishment Name Address ID/FEI Business Operations Regeneron Pharmaceuticals, Inc. 945589711 ANALYSIS(73604-914) , API MANUFACTURE(73604-914) Establishment Name Address ID/FEI Business Operations Kiniksa Pharmaceuticals (UK), Ltd. 224568777 MANUFACTURE(73604-914) Establishment Name Address ID/FEI Business Operations Jubilant HollisterStier LLC 069263643 ANALYSIS(73604-914) , MANUFACTURE(73604-914) Establishment Name Address ID/FEI Business Operations Packing Coordinators LLC 078525133 PACK(73604-914)
CloseEstablishment Name Address ID/FEI Business Operations Almac Pharma Services LLC 078607239 PACK(73604-914)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
RxNorm
ARCALYST- rilonacept injection, powder, lyophilized, for solution
Get Label RSS Feed for this Drug
ARCALYST- rilonacept injection, powder, lyophilized, for solution
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=ad965f38-dd22-4607-a673-2fb92f3b527a
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
ARCALYST- rilonacept injection, powder, lyophilized, for solution
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 73604-914-01 |
| 2 | 73604-914-04 |