The efficacy of SPINRAZA was demonstrated in two double-blind, sham-procedure controlled clinical trials in symptomatic infantile-onset and later-onset SMA patients (Study 1 and Study 2) and was ...
The efficacy of SPINRAZA was demonstrated in two double-blind, sham-procedure controlled clinical trials in symptomatic infantile-onset and later-onset SMA patients (Study 1 and Study 2) and was supported by open-label clinical trials conducted in presymptomatic and symptomatic SMA patients. The overall findings from these trials support the effectiveness of SPINRAZA across the range of SMA patients, and appear to support the early initiation of treatment with SPINRAZA.
14.1 Infantile-Onset SMA
Study 1 (NCT02193074) was a multicenter, randomized, double-blind, sham-procedure controlled study in 121 symptomatic infants ≤ 7 months of age at the time of first dose, diagnosed with SMA (symptom onset before 6 months of age). Patients were randomized 2:1 to receive either 12 mg SPINRAZA or sham injection as a series of loading doses administered intrathecally followed by maintenance doses administered every 4 months. Patients in this study were deemed most likely to develop Type 1 SMA.
A planned interim efficacy analysis was conducted based on patients who died, withdrew, or completed at least 183 days of treatment. Of the 82 patients included in the interim analysis (52 patients in the SPINRAZA-treated group and 30 in the sham-control group), 44% were male, 87% were Caucasian, 2% were Black, and 4% were Asian. Age at first treatment ranged from 30 to 262 days (median 181). Length of treatment ranged from 6 to 442 days (median 261 days). Baseline demographics were balanced between the SPINRAZA and control groups with the exception of age at first treatment (median age 175 vs. 206 days, respectively). The SPINRAZA and control groups were balanced with respect to gestational age, birth weight, disease duration, and SMN2 copy number. Median disease duration was 14 weeks. There was some imbalance in age at symptom onset with 88% of subjects in the SPINRAZA group and 77% in the control group experiencing symptoms within the first 12 weeks of life.
The primary endpoint assessed at the time of interim analysis was the proportion of responders: patients with an improvement in motor milestones according to Section 2 of the Hammersmith Infant Neurologic Exam (HINE). This endpoint evaluates seven different areas of motor milestone development, with a maximum score between 2-4 points for each, depending on the milestone, and a total maximum score of 26. A treatment responder was defined as any patient with at least a 2-point increase (or maximal score of 4) in ability to kick (consistent with improvement by at least 2 milestones), or at least a 1-point increase in the motor milestones of head control, rolling, sitting, crawling, standing or walking (consistent with improvement by at least 1 milestone). To be classified as a responder, patients needed to exhibit improvement in more categories of motor milestones than worsening. Of the 82 patients who were eligible for the interim analysis, a statistically significantly greater percentage of patients achieved the definition of a motor milestone responder in the SPINRAZA group (40%) compared to the sham-control group (0%). Results from the final analysis were consistent with those from the interim analysis (Table 3). Fifty-one percent of patients in the SPINRAZA group achieved the definition of a motor milestone responder compared to 0% of patients in the sham-control group. Figure 1 is a descriptive display of the distribution of net change from baseline in the total motor milestone score for Section 2 of the HINE for patients in the final efficacy set who did not die or withdraw from the study.
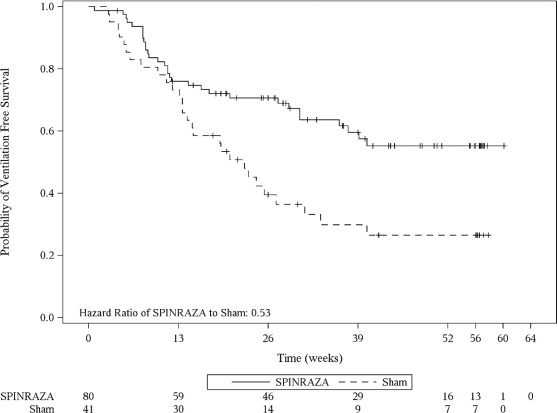
The primary endpoint assessed at the final analysis was time to death or permanent ventilation (≥ 16 hours ventilation/day continuously for > 21 days in the absence of an acute reversible event or tracheostomy). Statistically significant effects on event-free survival and overall survival were observed in patients in the SPINRAZA group compared to those in the sham-control group (Table 4). A 47% reduction in the risk of death or permanent ventilation was observed in the SPINRAZA group (p=0.005) (Figure 2). Median time to death or permanent ventilation was not reached in SPINRAZA group and was 22.6 weeks in the sham-control group. A statistically significant 63% reduction in the risk of death was also observed (p=0.004).
At the final analysis, the study also assessed treatment effects on the Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP-INTEND), which is an evaluation of motor skills in patients with infantile-onset SMA. The CHOP-INTEND results are displayed in Table 3.
Figure 1. Percent of Patients Who Died and Net Change from Baseline in Total Motor Milestone Score (HINE) Among Patients Alive in the Final Efficacy Set of Study 1 *
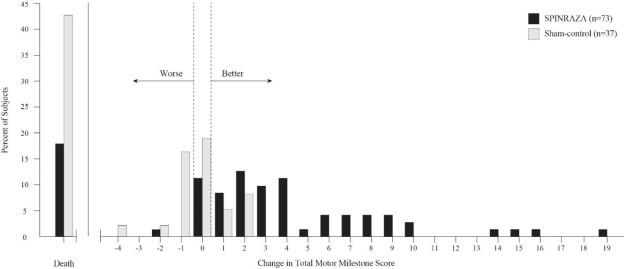
*For subjects who were alive and ongoing in the study, the change in total motor milestone score was calculated at the later of Day 183, Day 302, or Day 394.
Figure 2. Event-Free Survival in the Intent to Treat Set
14.2 Later-Onset SMA
Study 2 (NCT02292537) was a multicenter, randomized, double-blind, sham-procedure controlled study in 126 symptomatic children with later-onset SMA (symptom onset after 6 months of age). Patients were randomized 2:1 to either SPINRAZA 12 mg or sham injection as a series of loading doses administered intrathecally followed by maintenance doses administered every 6 months.
The median age at screening was 3 years (range 2-9 years), and the median age of onset of clinical signs and symptoms of SMA was 11 months (range 6-20 months). Of the 126 patients included in the study, 47% were male, 75% were Caucasian, 2% were Black, and 18% were Asian. Length of treatment ranged from 324 to 482 days (median 450 days). At baseline, patients had a mean Hammersmith Functional Motor Scale – Expanded (HFMSE) score of 21.6, all had achieved independent sitting, and no patients had achieved independent walking. Patients in this study were deemed most likely to develop Type 2 or 3 SMA.
The primary endpoint assessed was the change from baseline score at Month 15 on the HFMSE. The HFMSE evaluates motor function in patients with SMA who have limited ambulation, comprising of 33 scored activities that give objective information on motor ability and clinical progression, such as the ability to sit unassisted, stand, or walk. Each item is scored from 0-2, with a maximum total score of 66. Higher scores indicate better motor function. The primary analysis was conducted in the Intent to Treat (ITT) population, which included all subjects who were randomized and received at least 1 dose of SPINRAZA or at least one sham procedure. At the final analysis, a statistically significant improvement in HFMSE scores from baseline to Month 15 was observed in the SPINRAZA-treated group compared to the sham-control group (Table 5).
Figure 3. Mean Change from Baseline in HFMSE Score Over Time in the Intent to Treat Set1, 2(Study 2)
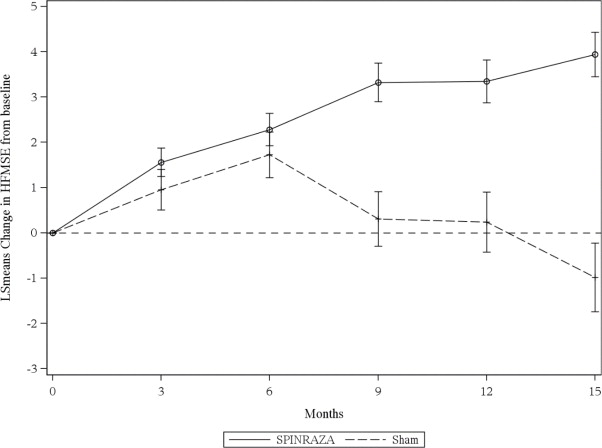
1Data for patients without a Month 15 visit were imputed using the multiple imputation method
2Error bars denote +/- standard error
14.3 Presymptomatic SMA
The results of the sham-controlled trial in infantile-onset (Study 1) (NCT02193074) and later-onset (Study 2) (NCT02292537) SMA patients were supported by an open-label uncontrolled trial conducted in 25 presymptomatic SMA patients who had a genetic diagnosis of 5q SMA and 2 or 3 copies of SMN2 (Study 3) (NCT02386553). In Study 3, 15 patients (60%) who had 2 SMN2 copies, and 10 patients (40%) who had 3 SMN2 copies; 48% were male, 56% were Caucasian, 12% were Asian, 4% were American Indian or Alaska Native, and 28% were of another race, or had no race reported. Patients ranged in age from 3 days to 42 days (median 22 days) at the time of first dose. Patients received 12 mg SPINRAZA as a series of loading doses administered intrathecally, followed by maintenance doses administered every 4 months. Patients were assessed with the World Health Organization (WHO) motor milestones, a set of 6 milestones in motor development that would be expected to be attained by 24 months of age in healthy children. An interim analysis was performed after all patients had received SPINRAZA for at least 14 months (median 25 months, range 14 to 34 months). Patients ranged in age from 14 to 34 months (median age of 26 months) at the time of the analysis. At the time of the interim analysis (data cutoff May 2018), all patients receiving SPINRAZA before the onset of SMA symptoms survived without requiring permanent ventilation, and beyond what would be expected based on their SMN2 copy number. All 25 patients (100%) had achieved the WHO motor milestone of sitting without support, and 22 patients (88%) had achieved the milestone of walking with assistance. Of the 22 patients who were older than the age expected to have achieved the ability to walk independently (as defined by the 95th percentile of the WHO expected age of achievement), 17 (77%) achieved the milestone of walking alone (i.e., walking independently).
Close