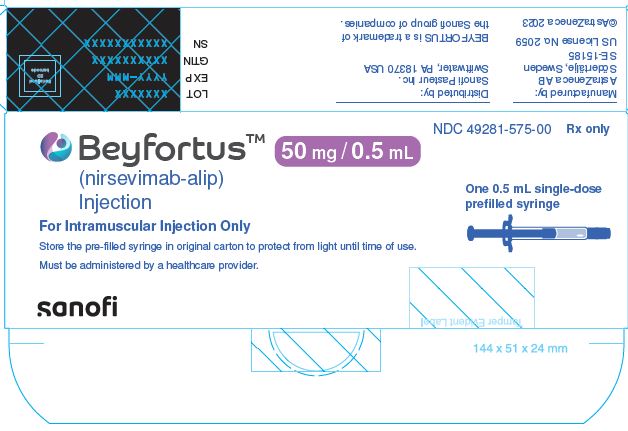
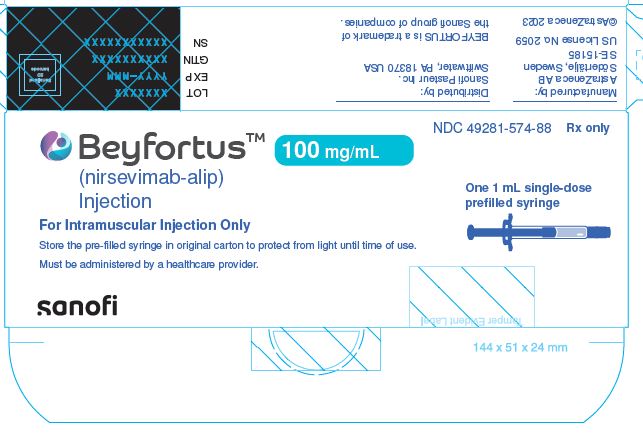
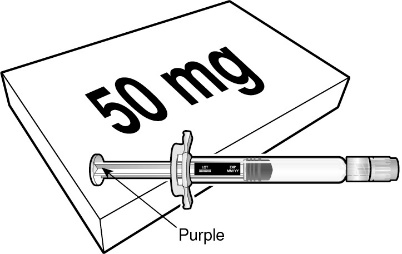
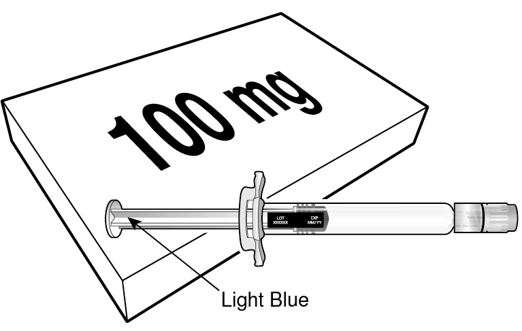
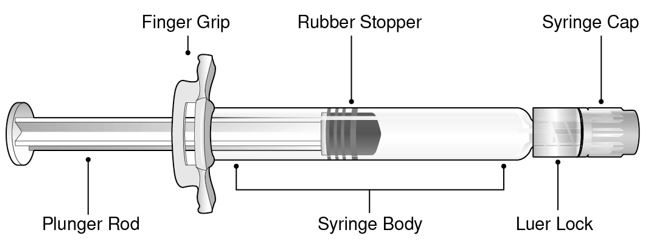
12.1 Mechanism of Action
- BEYFORTUS is a monoclonal antibody with anti-RSV activity [see Microbiology (12.4)].
12.2 Pharmacodynamics
- There is a positive correlation between a serum ...
12.1 Mechanism of Action
BEYFORTUS is a monoclonal antibody with anti-RSV activity [see Microbiology (12.4)].
12.2 Pharmacodynamics
There is a positive correlation between a serum nirsevimab-alip AUC (based on clearance at baseline) above 12.8 mg*day/mL and a lower incidence of medically attended RSV lower respiratory tract infection (MA RSV LRTI). Following IM administration of nirsevimab-alip in adults, RSV neutralizing antibody levels in serum were approximately 4 times higher than baseline at 8 hours after nirsevimab-alip dosing, and maximum levels were reached by day 6 following IM administration of nirsevimab-alip in adults. The safety and effectiveness of BEYFORTUS have not been established in adults.
Duration of Protection
Based on clinical data, the duration of protection offered by a single dose of BEYFORTUS extends through 5 months.
12.3 Pharmacokinetics
The PK of nirsevimab-alip is dose-proportional following a single IM administration of doses ranging from 25 mg (0.5 times the lowest approved recommended dosage) to 200 mg in pediatric subjects. Following the recommended dose, the nirsevimab-alip serum exposures were similar in neonates and infants born during or entering their first RSV season (Trials 03 and 04), and in neonates and infants born at less than 35 weeks GA (including less than 29 weeks GA) in their first RSV season (Trial 05), and in pediatric subjects up to 24 months of age with CLD or CHD in their first and second RSV season (Trial 05).
Absorption
The estimated nirsevimab-alip absolute bioavailability is 84% and the median time (range) to maximum concentration is 6 (1, 28) days.
Distribution
The estimated nirsevimab-alip total volume of distribution is 477 mL, for an infant weighing 5 kg.
Elimination
The nirsevimab-alip terminal half-life is approximately 71 days and the estimated clearance is 3.42 mL/day for an infant weighing 5 kg.
Metabolism
Nirsevimab-alip is degraded into small peptides by catabolic pathways.
Specific Populations
No clinically significant differences in the pharmacokinetics of nirsevimab-alip were observed based on race or vulnerability to severe RSV disease (i.e., CLD, CHD, GA <29 weeks).
The mean and median serum nirsevimab-alip concentrations in Trial 08 were lower than the concentrations in Trials 04 and 05. However, Trial 08 exposures were within the range shown to be effective in those who received the recommended dosage in Trials 03, 04, and 05 [see Pediatric Use (8.4)].
Drug Interaction Studies
No formal drug interaction studies have been performed with BEYFORTUS. Nirsevimab-alip is not predicted to be a substrate of, inhibitor or inducer of cytochrome P450 enzymes or transporter systems based on a mechanistic understanding of monoclonal antibodies.
Clinical Studies
Vaccines: There is limited experience with co-administration of BEYFORTUS with vaccines. In clinical trials, when BEYFORTUS was given concomitantly with routine childhood vaccines, the safety and reactogenicity profile of the co-administered regimen was similar to the childhood vaccines given alone.
12.4 Microbiology
Mechanism of Action
Nirsevimab-alip is a recombinant human IgG1κ monoclonal antibody that provides passive immunity by targeting the prefusion conformation of the RSV F protein. Nirsevimab-alip is long-acting due to a triple amino acid substitution (YTE) in the Fc region which increases binding to the neonatal Fc receptor and thereby extends serum half-life. Nirsevimab-alip binds to a conserved epitope in antigenic site Ø on the prefusion protein with dissociation constants KD = 0.12 nM and KD = 1.22 nM for RSV subtype A and B strains, respectively; it neutralizes RSV by inhibiting conformation changes in the F protein necessary for fusion of the viral and cellular membranes and viral entry.
Antiviral Activity
The cell culture neutralization activity of nirsevimab-alip against RSV was measured in a concentration-response model using cultured Hep-2 cells. Nirsevimab-alip neutralized clinical RSV isolates collected from global locations between 2003 and 2017 with median EC50 values for RSV A of 21 pM (3.2 ng/mL) (n=70; range 3 pM [0.48 ng/mL] to 100 pM [15 ng/mL]) and for RSV B of 19 pM (2.9 ng/mL) (n=49; range 2 pM [0.3 ng/mL] to 398 pM [59.7 ng/mL]).
Antiviral Resistance
In Cell Culture
Escape variants were selected following three passages in cell culture of RSV A2 and B9320 strains in the presence of nirsevimab-alip. Recombinant RSV A variants that showed reduced susceptibility to nirsevimab-alip compared with the reference strain included those with substitutions N67I+N208Y (103-fold reduction). Recombinant RSV B variants that showed reduced susceptibility to nirsevimab-alip included those with substitutions N208D (>90,000-fold change), N208S (>24,000-fold change), K68N+N201S (>13,000-fold change), and K68N+N208S (>90,000-fold change). All resistance-associated substitutions identified among neutralization escape variants were located in the nirsevimab-alip binding site (amino acids 62-69 and 196-212) and were shown to reduce binding affinity to RSV F protein.
In Surveillance Trials
Polymorphisms conferring large fold-reductions in susceptibility to nirsevimab-alip in isolates collected from 1956-2014 were not observed for RSV A and seen rarely (<1%) for RSV B, and included K65Q+K68N (1,239-fold change), K65Q+S211N (36-fold change), and L203I (3,005-fold change) substitutions. In prospective, observational, global molecular epidemiology studies (OUTSMART-RSV and INFORM-RSV) genetic diversity of RSV F protein sequences has remained low (most amino acids in both RSV A and RSV B >99% conserved). Variants harboring known nirsevimab-alip resistance-associated substitutions have been rare (<1%) and include RSV B substitutions K68Q (>369-fold change), N201T (>406-fold change) and N201T+I206M+Q209R (>418-fold change). Variants observed with reduced susceptibility include RSV A substitutions K68E (13-fold change), K68N (5-fold change), and S275F (6-fold change), and RSV B substitutions K68N (30-fold change), K68Q+I206M+Q209R (46-fold change), N201S (127-fold change) and N201S+I206M+Q209R (17-fold change). The clinical significance of these reductions in susceptibility is not known. From 2015 to 2021, most amino acid residues in the nirsevimab-alip binding site were highly conserved (>99%) at all positions in RSV A and 22 of the 25 positions in RSV B. Co-occurring substitutions I206M+Q209R in the nirsevimab-alip binding site that have become prevalent in RSV B since 2017 did not confer reduced susceptibility (<5-fold change) to nirsevimab-alip. The S211N substitution which has increased in prevalence also retains susceptibility to nirsevimab-alip, both individually and concurrently with I206M+Q209R.
In Clinical Trials
In Trial 04, Trial 05 and Trial 08 no known resistance-associated substitutions were identified at ≥25% frequency at any sampling time points. Phenotypic testing of novel substitutions is ongoing.
In Trial 03 (who received a single dose of 50 mg BEYFORTUS), 2 of 40 subjects with RSV infections corresponding to any case definition had a variant containing nirsevimab-alip resistance-associated substitutions. The two subjects received less than the recommended nirsevimab-alip dose and had RSV B variants harboring I64T+K68E+I206M+Q209R co-occurring substitutions or an N208S substitution. I64T, K68E, and N208S substitutions individually have reduced susceptibility to nirsevimab-alip (fold changes: >496, >283, and >387, respectively).
In Trial 04, an RSV B variant harboring binding site substitution L204S (no phenotypic data) concurrent with I206M+Q209R+S211N substitutions (<5-fold change) was detected at ≥25% frequency in one subject who received BEYFORTUS through Day 150. RSV B variants present at <25% frequency with I64T+K68E substitutions (>280-fold-change) and N208I substitution (>600-fold change) were seen in one subject each who received BEYFORTUS through Day 150. In addition, an RSV B N201D substitution (no phenotypic data) was observed at 9% frequency in one subject after Day 150.
Cross-resistance
Limited data are available that show that variants resistant to nirsevimab-alip could have cross-resistance to palivizumab. Palivizumab retained full neutralization potency against resistance-associated substitutions identified in Trial 03 and Trial 04. Nirsevimab-alip retained activity against recombinant RSV harboring palivizumab resistance-associated substitutions identified in molecular epidemiology studies and in neutralization escape variants of palivizumab; S275F substitution had reduced susceptibility of 6-fold.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies (ADA) is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of ADA in the studies described below with the incidence of ADA in other studies, including those of nirsevimab-alip or of other nirsevimab products.
Of 572 subjects receiving the recommended dose of nirsevimab-alip in Trial 03, 3.3% (16/492) subjects were ADA-positive on Day 361. Among the 16 ADA-positive subjects, 94% (15/16) had ADA against YTE and none tested positive for neutralizing antibodies against nirsevimab-alip.
In Trial 04, 5% (95/1778) of subjects were ADA-positive on Day 361, of whom 21% (20/95) had neutralizing antibodies and 77% (73/95) had ADA against YTE.
In Trial 05, among subjects enrolled during their first RSV season, on Day 361, 6% (32/538) of subjects were positive for ADA. Among the 32 ADA-positive subjects, 6% (2/32) had neutralizing antibodies against nirsevimab-alip and 91% (29/32) had antibodies against the YTE substitution. Of 180 subjects who received nirsevimab-alip in two consecutive RSV seasons, on season 2 Day 361, 9% (13/144) of subjects were positive for ADA. Among the 13 ADA-positive subjects, 8% (1/13) had neutralizing antibodies against nirsevimab-alip and 62% (8/13) had antibodies against YTE.
In Trial 08, among subjects receiving nirsevimab-alip in their first or second RSV season, 13% (9/67) of subjects were positive for ADA on Day 361. Among the 9 ADA-positive subjects, 11% (1/9) had neutralizing antibodies against nirsevimab-alip and 100% (9/9) were positive for ADA against YTE.
In Trials 03, 04, 05, and 08 the effect of ADA on nirsevimab-alip serum concentrations through Day 151 could not be determined. Subjects who received BEYFORTUS who developed anti-nirsevimab-alip antibodies had reduced nirsevimab-alip concentrations at Day 361 (40% to 60% lower compared to subjects who received BEYFORTUS who did not develop anti-nirsevimab-alip antibodies). Because of the low occurrence of ADA and MA RSV LRTI in clinical trials, the effect of these ADA on effectiveness of BEYFORTUS is unknown.
Close