Label: MOLINDONE HYDROCHLORIDE tablet
- NDC Code(s): 42806-336-01, 42806-337-01, 42806-338-01
- Packager: EPIC PHARMA, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
WARNING
Increased Mortality in Elderly Patients with Dementia-Related Psychosis – Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Molindone Hydrochloride Tablets, USP are not approved for the treatment of patients with dementia-related psychosis (see WARNINGS).
Close -
DESCRIPTION
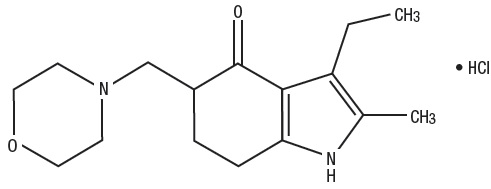
Molindone Hydrochloride is a dihydroindolone compound which is not structurally related to the phenothiazines, the butyrophenones or the thioxanthenes. Molindone Hydrochloride is 3-ethyl-6 ...
-
CLINICAL PHARMACOLOGY Molindone Hydrochloride has a pharmacological profile in laboratory animals which predominantly resembles that of other antipsychotic agents causing reduction of spontaneous locomotion and ...
-
INDICATIONS AND USAGE Molindone Hydrochloride Tablets, USP are indicated for the management of schizophrenia. The efficacy of Molindone Hydrochloride Tablets, USP in schizophrenia was established in clinical studies ...
-
CONTRAINDICATIONS Molindone Hydrochloride Tablets are contraindicated in severe central nervous system depression (alcohol, barbiturates, narcotics, etc.) or comatose states, and in patients with known ...
-
WARNINGS Increased Mortality in Elderly Patients with Dementia-Related Psychosis — Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of ...
-
PRECAUTIONS General - Some patients receiving Molindone Hydrochloride may note drowsiness initially and they should be advised against activities requiring mental alertness until their response to the drug ...
-
ADVERSE REACTIONS CNS Effects - The most frequently occurring effect is initial drowsiness that generally subsides with continued usage of the drug or lowering of the dose. Noted less frequently were depression ...
-
OVERDOSAGE Symptomatic, supportive therapy should be the rule. Gastric lavage is indicated for the reduction of absorption of Molindone Hydrochloride which is freely soluble in water. Since the adsorption of ...
-
DOSAGE AND ADMINISTRATION Initial and maintenance doses of Molindone Hydrochloride Tablets should be individualized. Initial Dosage Schedule - The usual starting dosage is 50 to 75 mg/day. —Increase to 100 mg/day in 3 or ...
-
HOW SUPPLIED Molindone Hydrochloride Tablets USP, 5 mg are light orange to orange, oval-shaped tablets, debossed "Є336" on one side and plain on the other side. They are supplied as follows: Bottles of 100 ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Molindone 5 mg 100ct

-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Molindone 10 mg 100ct

-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – Molindone 25 mg 100ct

-
INGREDIENTS AND APPEARANCEProduct Information