Label: CAMZYOS- mavacamten capsule, gelatin coated
- NDC Code(s): 73625-111-11, 73625-112-11, 73625-113-11, 73625-114-11
- Packager: Myokardia, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CAMZYOS safely and effectively. See full prescribing information for CAMZYOS. CAMZYOS® (mavacamten) capsules for oral use - Initial ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: RISK OF HEART FAILURE
CAMZYOS reduces left ventricular ejection fraction (LVEF) and can cause heart failure due to systolic dysfunction [see Warnings and Precautions (5.1)].
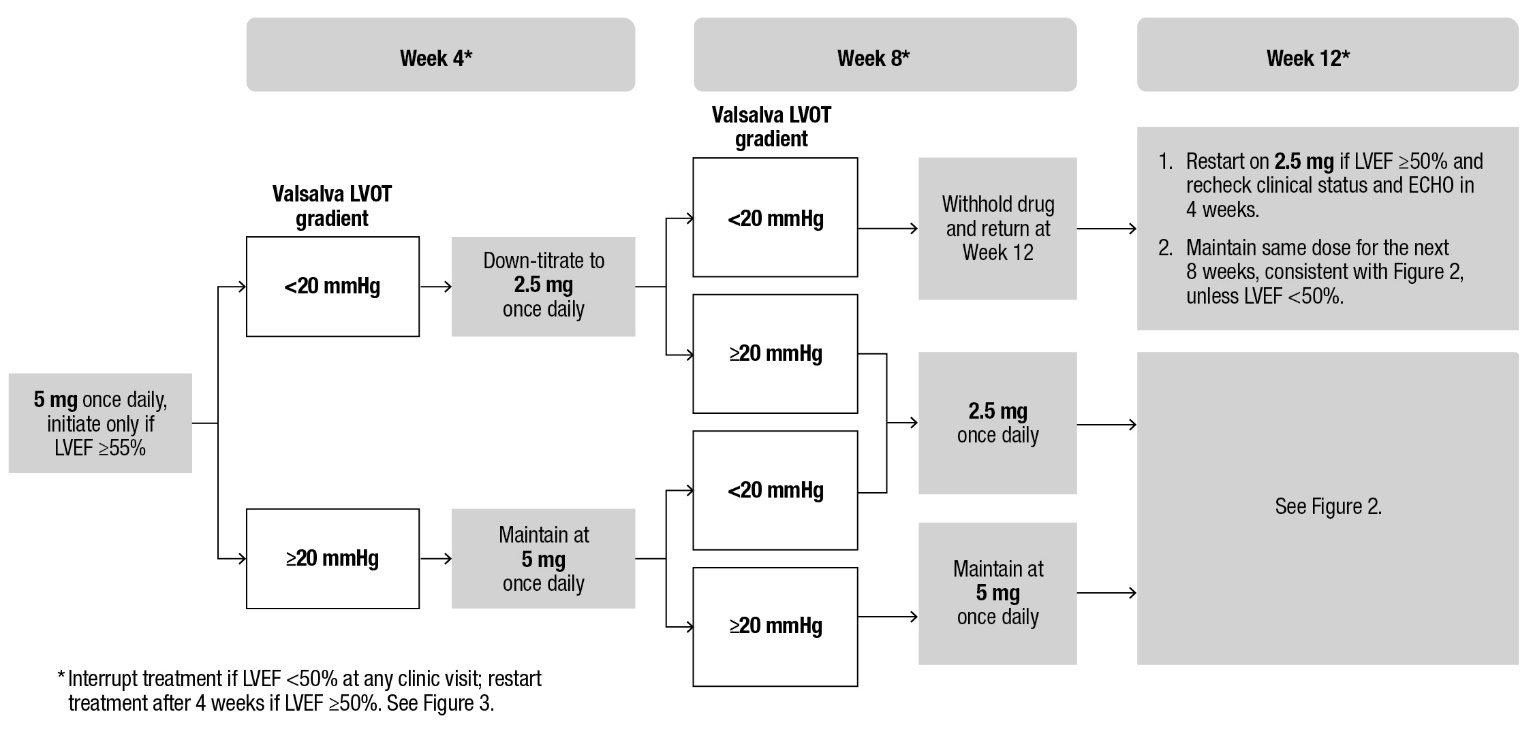
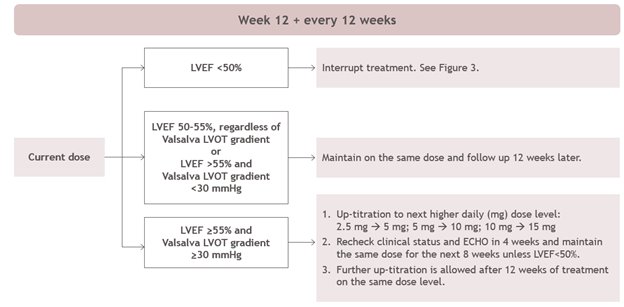
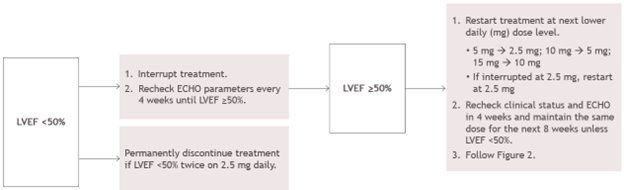
Echocardiogram assessments of LVEF are required prior to and during treatment with CAMZYOS. Initiation of CAMZYOS in patients with LVEF <55% is not recommended. Interrupt CAMZYOS if LVEF is <50% at any visit or if the patient experiences heart failure symptoms or worsening clinical status [see Dosage and Administration (2.1) and Warnings and Precautions (5.1)].
Concomitant use of CAMZYOS with certain cytochrome P450 inhibitors or discontinuation of certain cytochrome P450 inducers may increase the risk of heart failure due to systolic dysfunction; therefore, the use of CAMZYOS is contraindicated with the following [see Contraindications (4) and Warnings and Precautions (5.2)]:
- •
- Moderate to strong CYP2C19 inhibitors or strong CYP3A4 inhibitors
- •
- Moderate to strong CYP2C19 inducers or moderate to strong CYP3A4 inducers
Because of the risk of heart failure due to systolic dysfunction, CAMZYOS is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called CAMZYOS REMS PROGRAM [see Warnings and Precautions (5.3)].
Close -
1 INDICATIONS AND USAGE CAMZYOS® is indicated for the treatment of adults with symptomatic New York Heart Association (NYHA) class II-III obstructive hypertrophic cardiomyopathy (HCM) to improve functional capacity and ...
-
2 DOSAGE AND ADMINISTRATION 2.1 Initiation, Maintenance, and Interruption of Treatment - Confirm absence of pregnancy and usage of effective contraception in females of reproductive potential [see Warnings and Precautions ...
-
3 DOSAGE FORMS AND STRENGTHS CAMZYOS is available as capsules imprinted with the strength and “Mava” in the following strengths: • 2.5 mg – light purple cap - • 5 mg – yellow cap - • 10 mg – pink cap - • 15 mg – gray cap
-
4 CONTRAINDICATIONS CAMZYOS is contraindicated with concomitant use of: • Moderate to strong CYP2C19 inhibitors or strong CYP3A4 inhibitors [see Warnings and Precautions (5.2), Drug Interactions (7.1)] • Moderate ...
-
5 WARNINGS AND PRECAUTIONS 5.1 Heart Failure - CAMZYOS reduces systolic contraction and can cause heart failure or totally block ventricular function. Patients who experience a serious intercurrent illness (e.g., serious ...
-
6 ADVERSE REACTIONS The following adverse reaction is discussed in other sections of the labeling: • Heart failure [see Warnings and Precautions (5.1)] 6.1 Clinical Trials Experience - Because clinical trials ...
-
7 DRUG INTERACTIONS 7.1 Potential for Other Drugs to Affect Plasma Concentrations of CAMZYOS - Mavacamten is primarily metabolized by CYP2C19 and to a lesser extent by CYP3A4 and CYP2C9. Inducers and inhibitors of ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Based on animal data, CAMZYOS may cause fetal harm when administered to a pregnant female. There are no human data on the use of CAMZYOS during pregnancy to ...
-
10 OVERDOSAGE Clinical Experience and Effects - • Cardiovascular effects may include reduced LVEF (left ventricular ejection fraction), heart failure, hypotension, and asystole refractory to medical ...
-
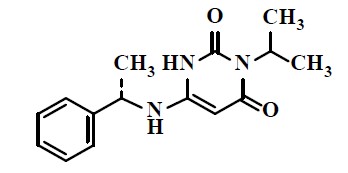
11 DESCRIPTION CAMZYOS capsules for oral use contain mavacamten, a cardiac myosin inhibitor. The chemical name of mavacamten is 3-(1-methylethyl)-6-[[(1S)-1-phenylethyl]amino]-2,4(1H,3H)-pyrimidinedione. The ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Mavacamten is an allosteric and reversible inhibitor selective for cardiac myosin. Mavacamten modulates the number of myosin heads that can enter “on actin ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility - Mavacamten was not genotoxic in a bacterial reverse mutation test (Ames test), a human in vitro lymphocyte clastogenicity assay ...
-
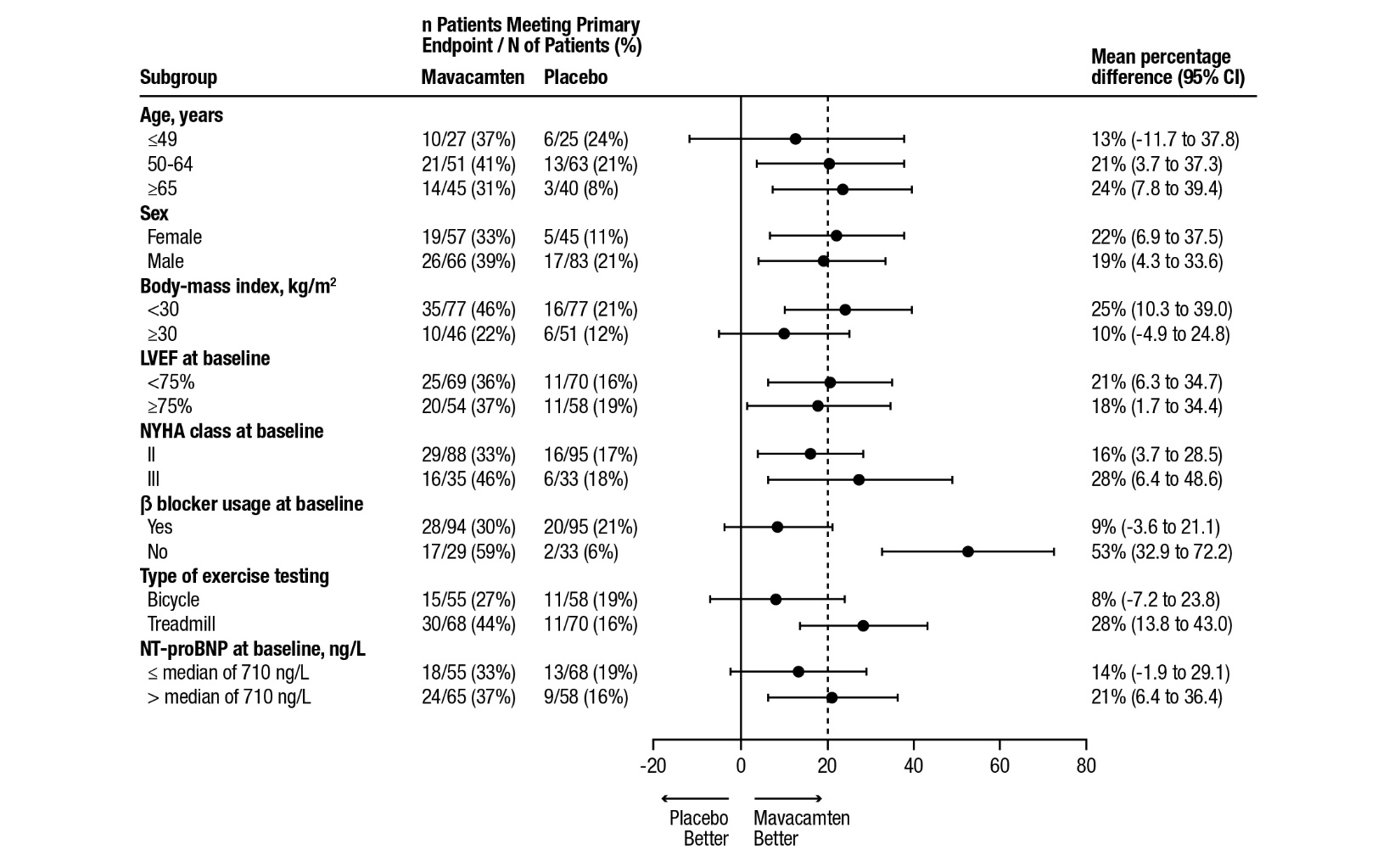
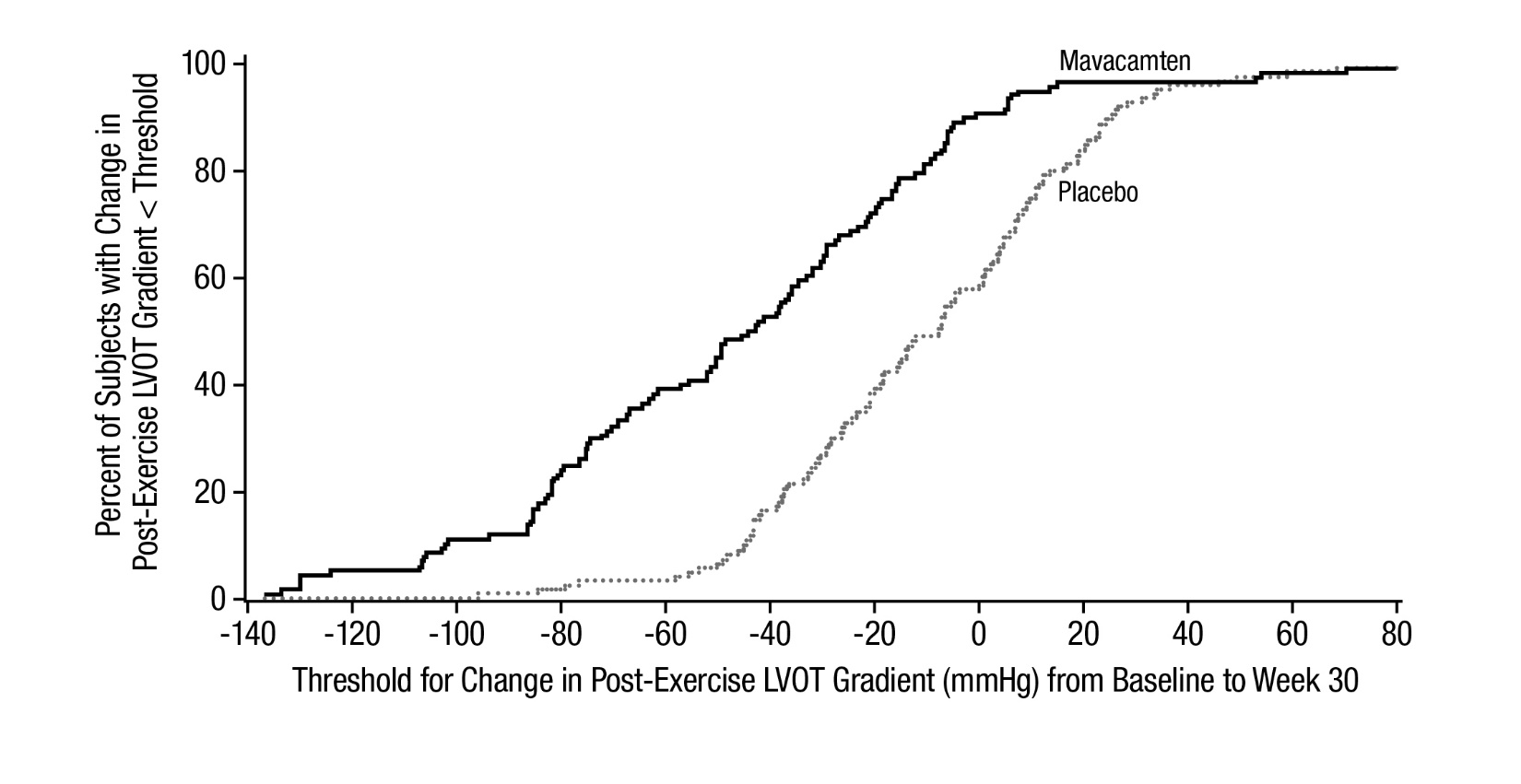
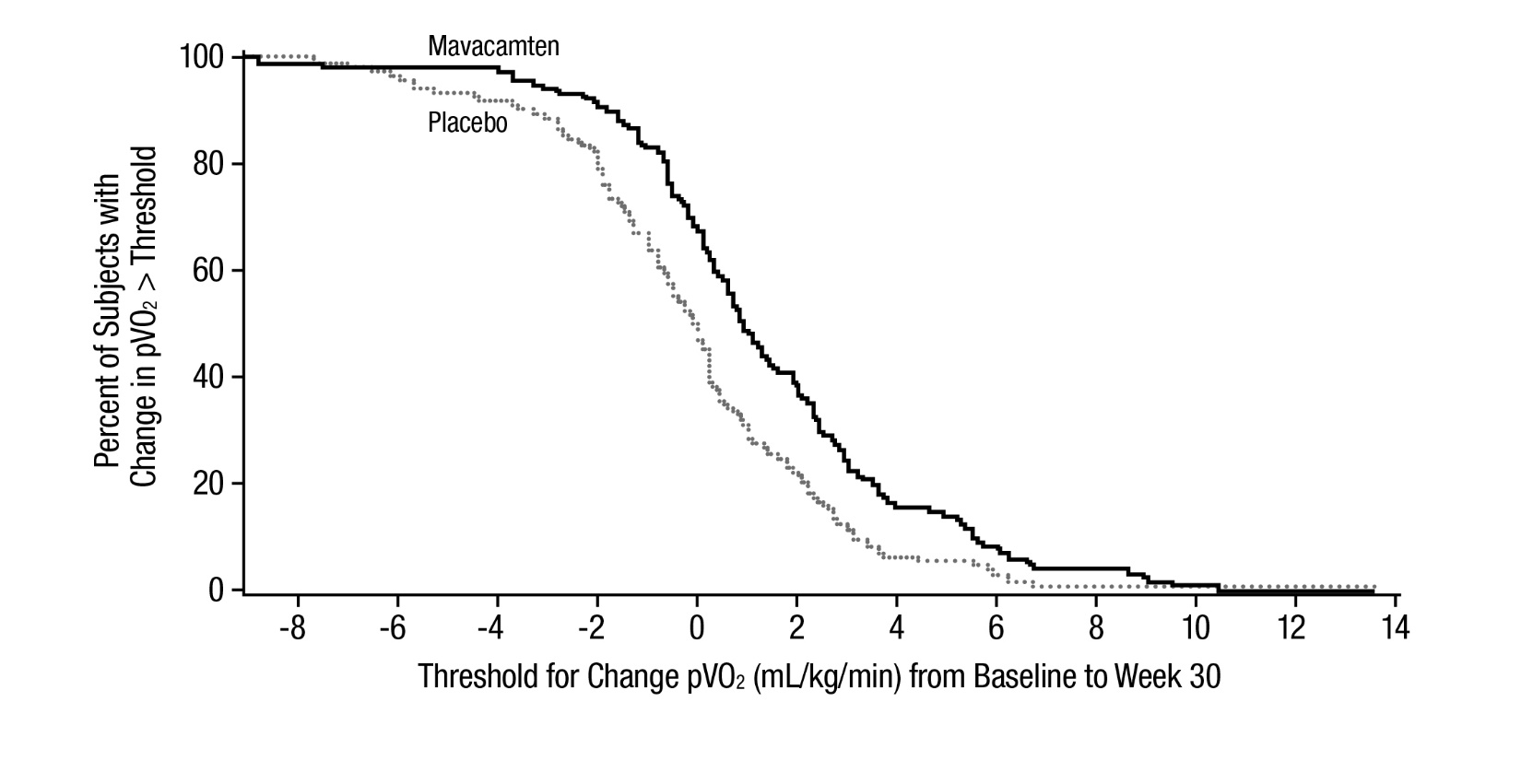
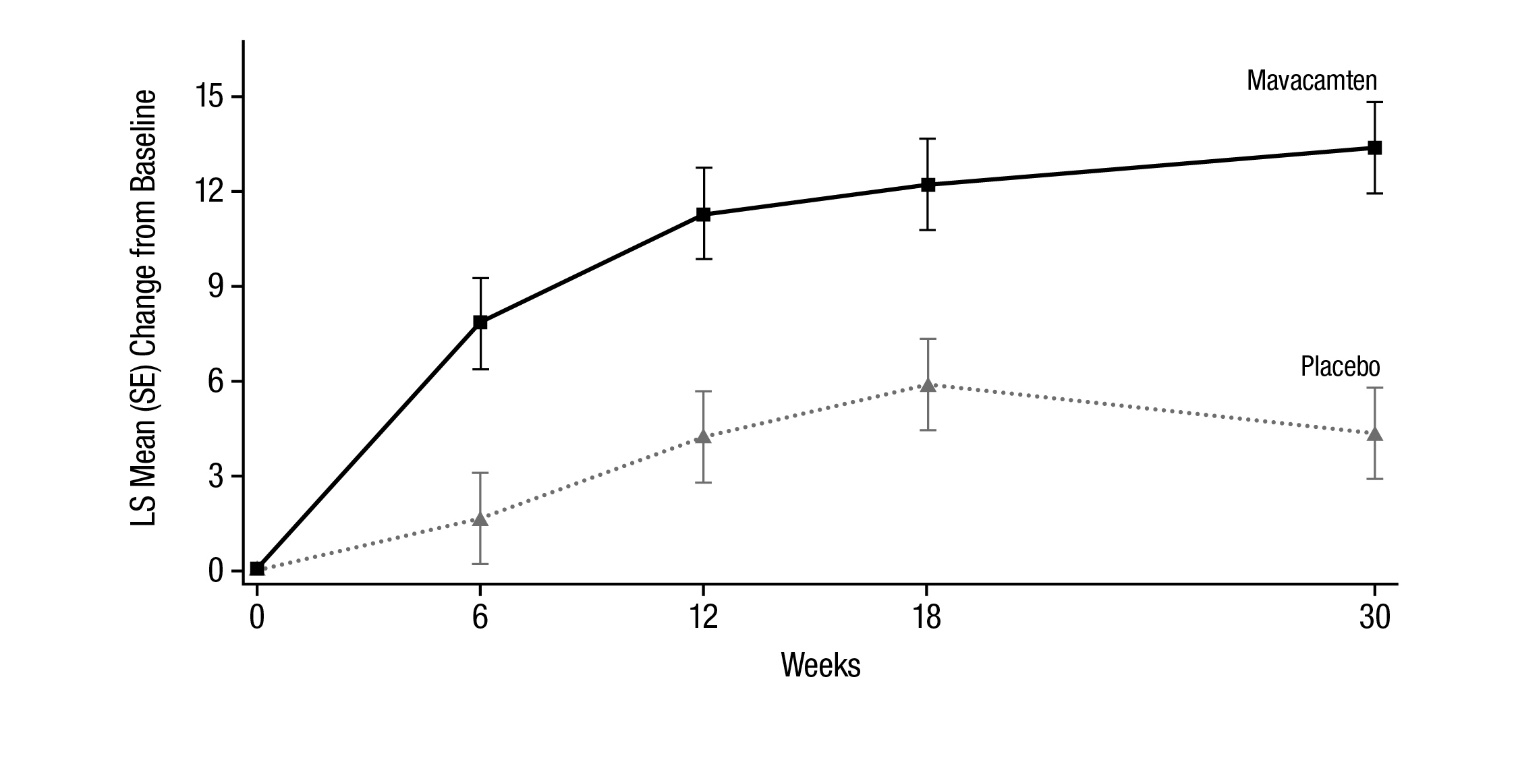
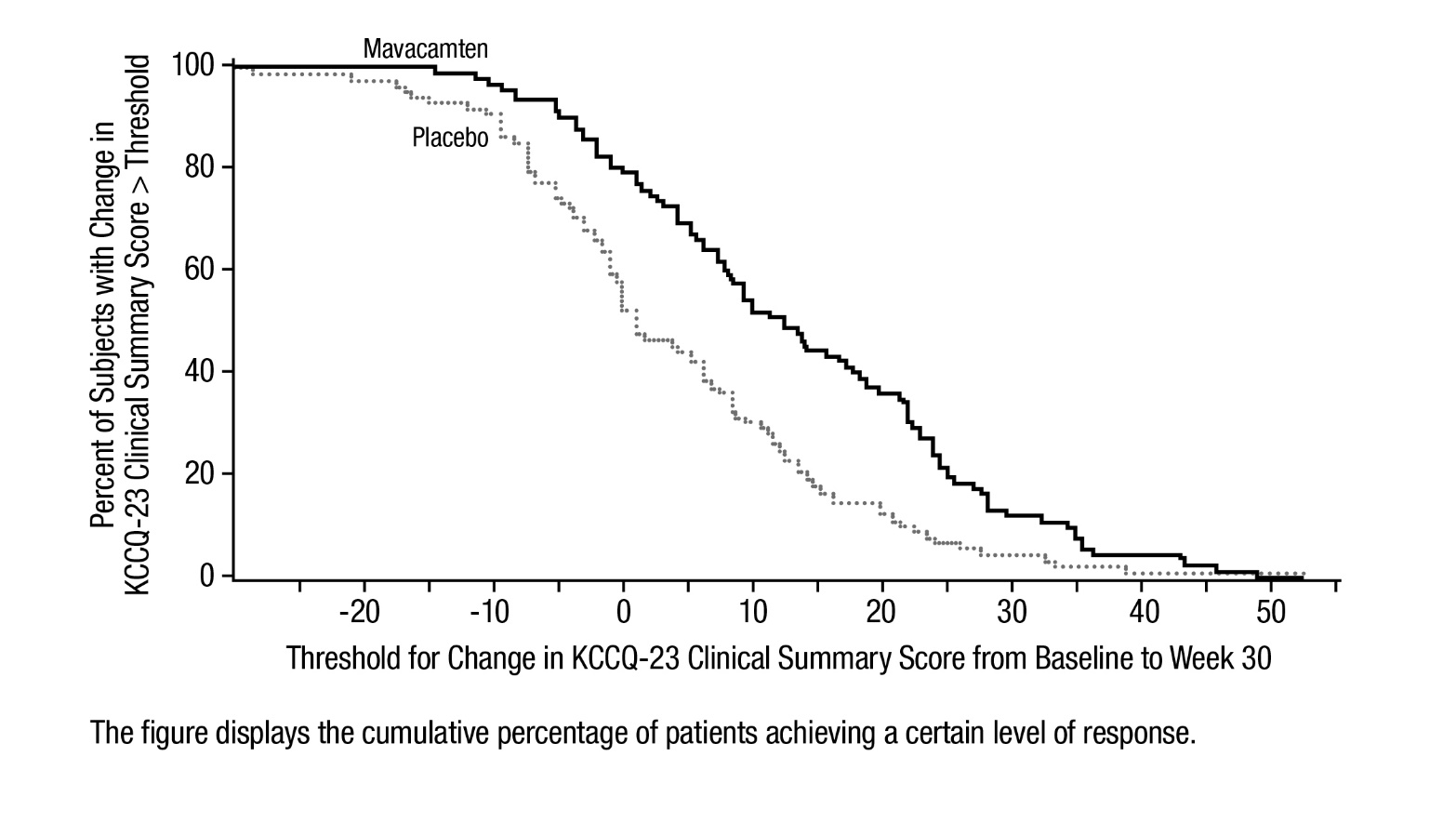
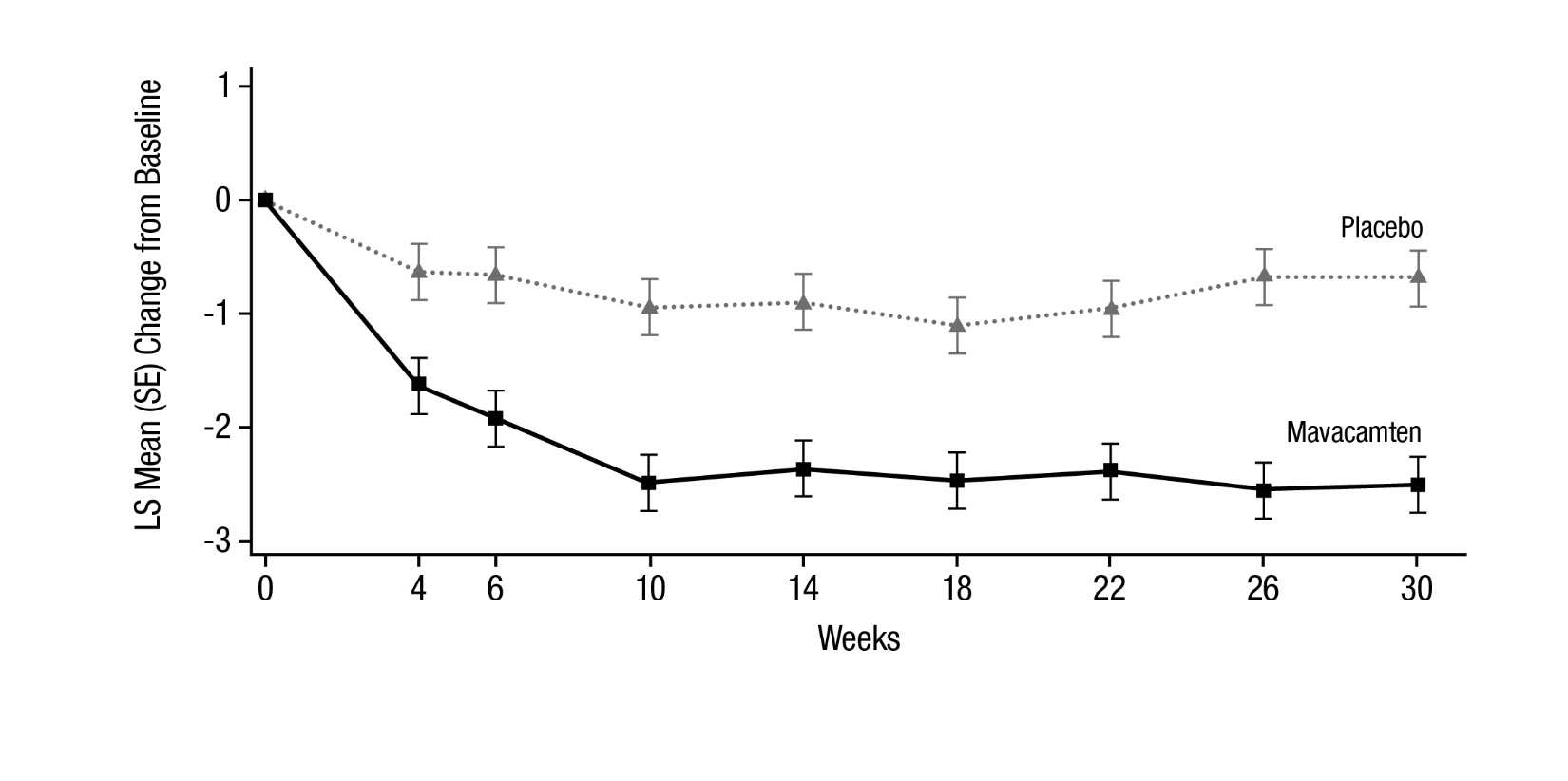
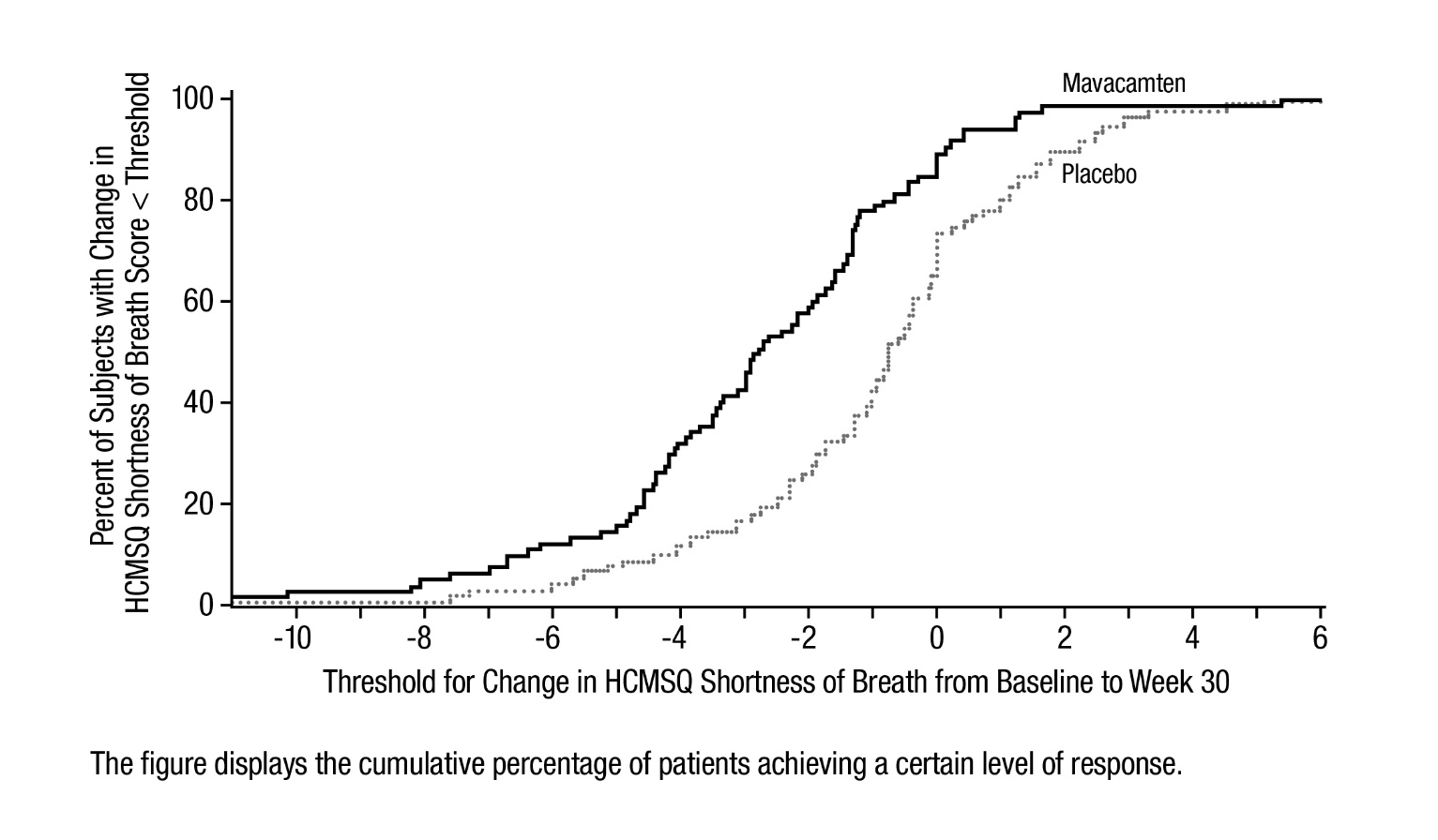
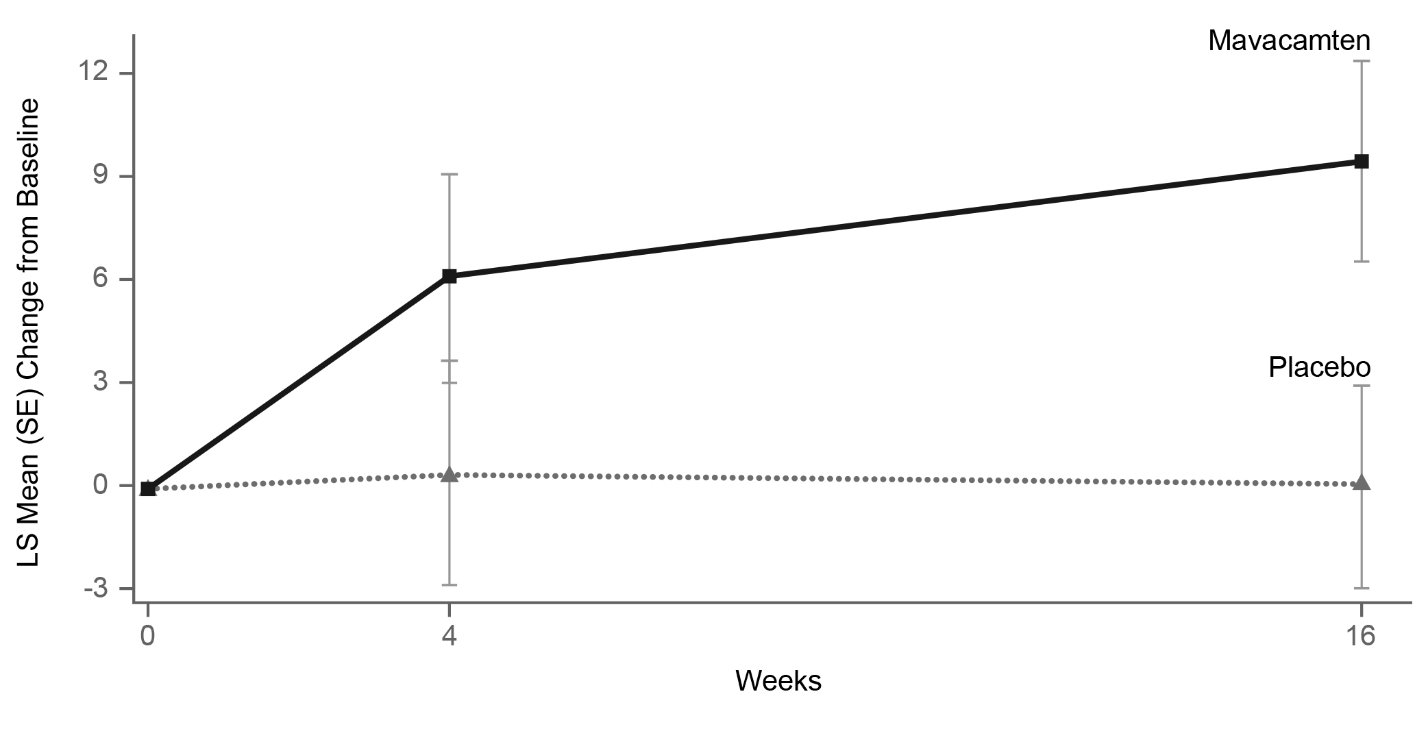
14 CLINICAL STUDIES EXPLORER-HCM - The efficacy of CAMZYOS was evaluated in EXPLORER-HCM (NCT-03470545) a Phase 3, double-blind, randomized, placebo-controlled, multicenter, international, parallel-group trial in 251 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING CAMZYOS® is supplied as immediate release Size 2 hard gelatin capsules containing 2.5 mg, 5 mg, 10 mg, or 15 mg of mavacamten. White opaque capsule bodies are imprinted with “Mava”, and the opaque ...
-
17 PATIENT COUNSELING INFORMATION Advise the patient and/or caregiver to read the FDA-approved patient labeling (Medication Guide). Heart Failure - Inform patients that cardiac function monitoring must be performed using ...
-
Medication Guide MEDICATION GUIDE - CAMZYOS® (kam-zai-ōs) (mavacamten) capsules, for oral use - What is the most important information I should know about CAMZYOS? CAMZYOS may cause serious side ...
-
CAMZYOS 2.5 mg Label NDC 73625-111-11 - Rx only - CAMZYOS™ (mavacamten) capsules - 2.5 mg - Dispense the enclosed Medication Guide to each patient. Recommended Dosage: See Prescribing Information - Keep out of reach of ...
-
CAMZYOS 5 mg Label NDC 73625-112-11 - Rx only - CAMZYOS™ (mavacamten) capsules - 5 mg - Dispense the enclosed Medication Guide to each patient. Recommended Dosage: See Prescribing Information - Keep out of reach of ...
-
CAMZYOS 10 mg Label NDC 73625-113-11 - Rx only - CAMZYOS™ (mavacamten) capsules - 10 mg - Dispense the enclosed Medication Guide to each patient. Recommended Dosage: See Prescribing Information - Keep out of reach of ...
-
CAMZYOS 15 mg Label NDC 73625-114-11 - Rx only - CAMZYOS™ (mavacamten) capsules - 15 mg - Dispense the enclosed Medication Guide to each patient. Recommended Dosage: See Prescribing Information - Keep out of reach of ...
-
INGREDIENTS AND APPEARANCEProduct Information