Label: SUNLENCA- lenacapavir sodium tablet, film coated
SUNLENCA- lenacapavir sodium kit
- NDC Code(s): 61958-3001-1, 61958-3001-2, 61958-3001-3, 61958-3002-1, view more
- Packager: Gilead Sciences, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 26, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SUNLENCA safely and effectively. See full prescribing information for SUNLENCA. SUNLENCA® (lenacapavir) tablets, for oral use ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGESUNLENCA, in combination with other antiretroviral(s), is indicated for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in heavily treatment-experienced adults with ...
-
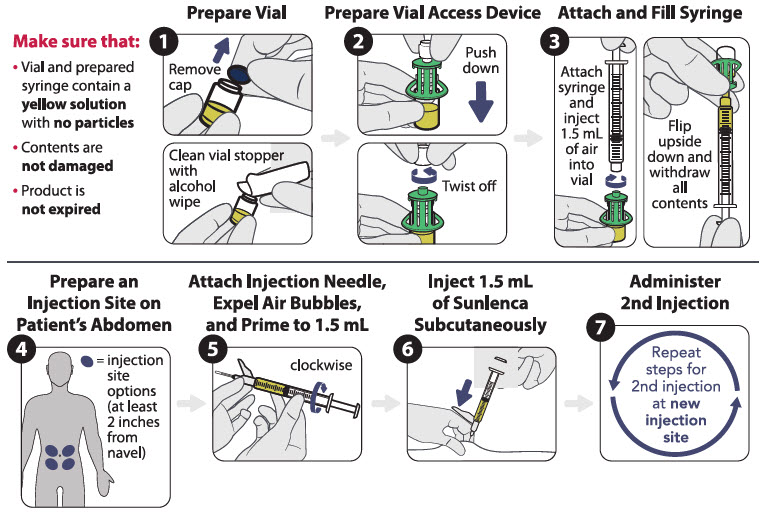
2 DOSAGE AND ADMINISTRATION2.1 Adherence to Treatment Regimen - Prior to starting SUNLENCA, healthcare providers should carefully select patients who agree to the required every 6 month injection dosing schedule and ...
-
3 DOSAGE FORMS AND STRENGTHSSUNLENCA tablets: Each tablet contains 300 mg of lenacapavir (present as 306.8 mg of lenacapavir sodium). The tablets are beige, capsule-shaped, film-coated, and debossed with 'GSI' on one side ...
-
4 CONTRAINDICATIONSConcomitant administration of SUNLENCA with strong CYP3A inducers is contraindicated due to decreased lenacapavir plasma concentrations, which may result in the loss of therapeutic effect and ...
-
5 WARNINGS AND PRECAUTIONS5.1 Immune Reconstitution Syndrome - Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy. During the initial phase of combination ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in other sections of the labeling: Immune Reconstitution Syndrome [see Warnings and Precautions (5.1)] Injection Site Reactions [see Warnings and ...
-
7 DRUG INTERACTIONS7.1 Effect of Other Drugs on SUNLENCA - Lenacapavir is a substrate of P-gp, UGT1A1, and CYP3A. Strong or Moderate CYP3A Inducers - Drugs that are strong or moderate inducers of CYP3A may ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in individuals exposed to SUNLENCA during pregnancy. Healthcare providers ...
-
10 OVERDOSAGENo data are available on overdose of SUNLENCA in patients. If overdose occurs, monitor the patient for evidence of toxicity. Treatment of overdose with SUNLENCA consists of general supportive ...
-
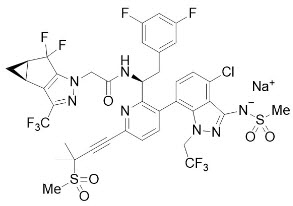
11 DESCRIPTIONSUNLENCA tablets and SUNLENCA injection contain lenacapavir sodium, a capsid inhibitor. The chemical name of lenacapavir sodium is: Sodium ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - SUNLENCA is an HIV-1 antiretroviral agent [see Microbiology (12.4)]. 12.2 Pharmacodynamics - Exposure-Response - In CAPELLA, oral loading doses (600 mg on Day 1 ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Lenacapavir was not carcinogenic in a 6-month rasH2 transgenic mouse study in males or females at doses of up to ...
-
14 CLINICAL STUDIESThe efficacy and safety of SUNLENCA in heavily treatment-experienced participants with multidrug resistant HIV-1 is based on 52-week data from CAPELLA, a randomized, placebo-controlled ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSUNLENCA tablets, 300 mg are beige, capsule-shaped, and film-coated with "GSI" debossed on one side and "62L" on the other side. SUNLENCA tablets are available in a bottle and blister packs ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information). Drug Interactions - SUNLENCA may interact with certain drugs; therefore, advise patients to report to their ...
-
SPL UNCLASSIFIED SECTIONSUNLENCA is a trademark of Gilead Sciences, Inc., or its related companies. All other trademarks referenced herein are the property of their respective owners. © 2024 Gilead Sciences, Inc. All ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - SUNLENCA® (sun-LEN-kuh) (lenacapavir) tablets SUNLENCA® (sun-LEN-kuh) (lenacapavir) injection - This Patient Information has been approved by the U.S. Food and Drug ...
-
PRINCIPAL DISPLAY PANEL - 300 mg Tablet Blister Pack Pouch CartonNDC 61958-3001-1 - Sunlenca® (lenacapavir) tablets - 300 mg per tablet - 1 pouch containing 4 tablets - Each tablet contains: 300 mg of lenacapavir - (present as 306.8 mg lenacapavir sodium). Store at 20 ...
-
PRINCIPAL DISPLAY PANEL - 5 Tablet Blister Pack Pouch CartonNDC 61958-3001-2 - Sunlenca® (lenacapavir) tablets - 300 mg per tablet - 1 pouch containing 5 tablets - Each tablet contains: 300 mg of lenacapavir - (present as 306.8 mg lenacapavir sodium). Store at 20 ...
-
PRINCIPAL DISPLAY PANEL - 4 Tablet Bottle LabelNDC 61958-3001-3 - 4 tablets - Sunlenca® (lenacapavir) tablets - 300 mg per tablet - Talk to your healthcare provider - before taking Sunlenca tablets. Your healthcare provider will tell you - when to take ...
-
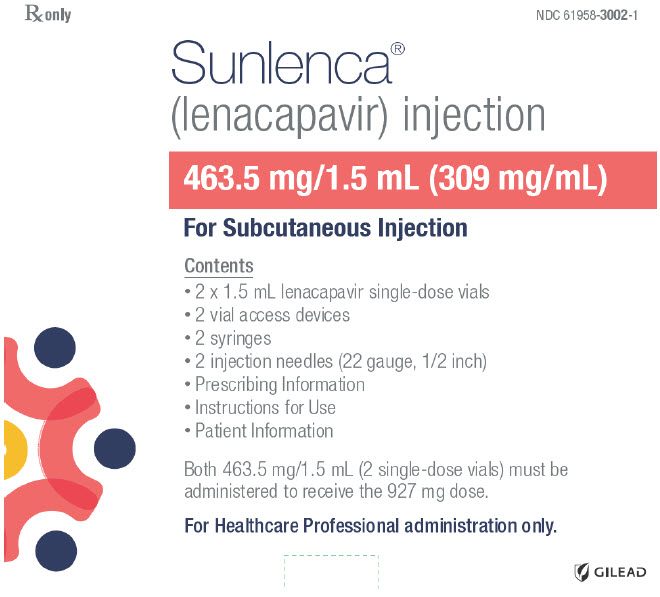
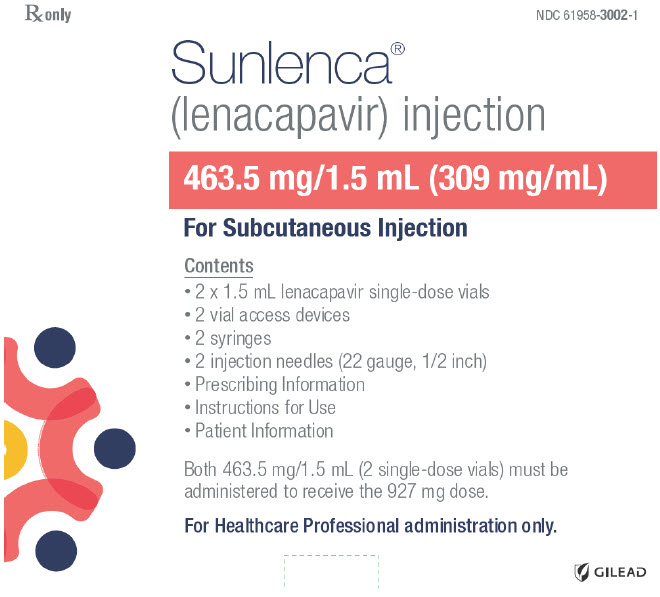
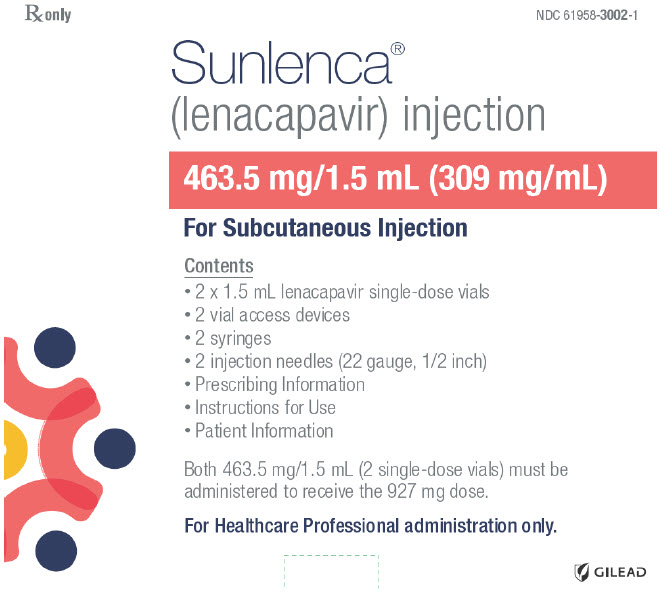
PRINCIPAL DISPLAY PANEL - Kit CartonRx only - NDC 61958-3002-1 - Sunlenca® (lenacapavir) injection - 463.5 mg/1.5 mL (309 mg/mL) For Subcutaneous Injection - Contents - 2 x 1.5 mL lenacapavir single-dose vials - 2 vial access devices - 2 ...
-
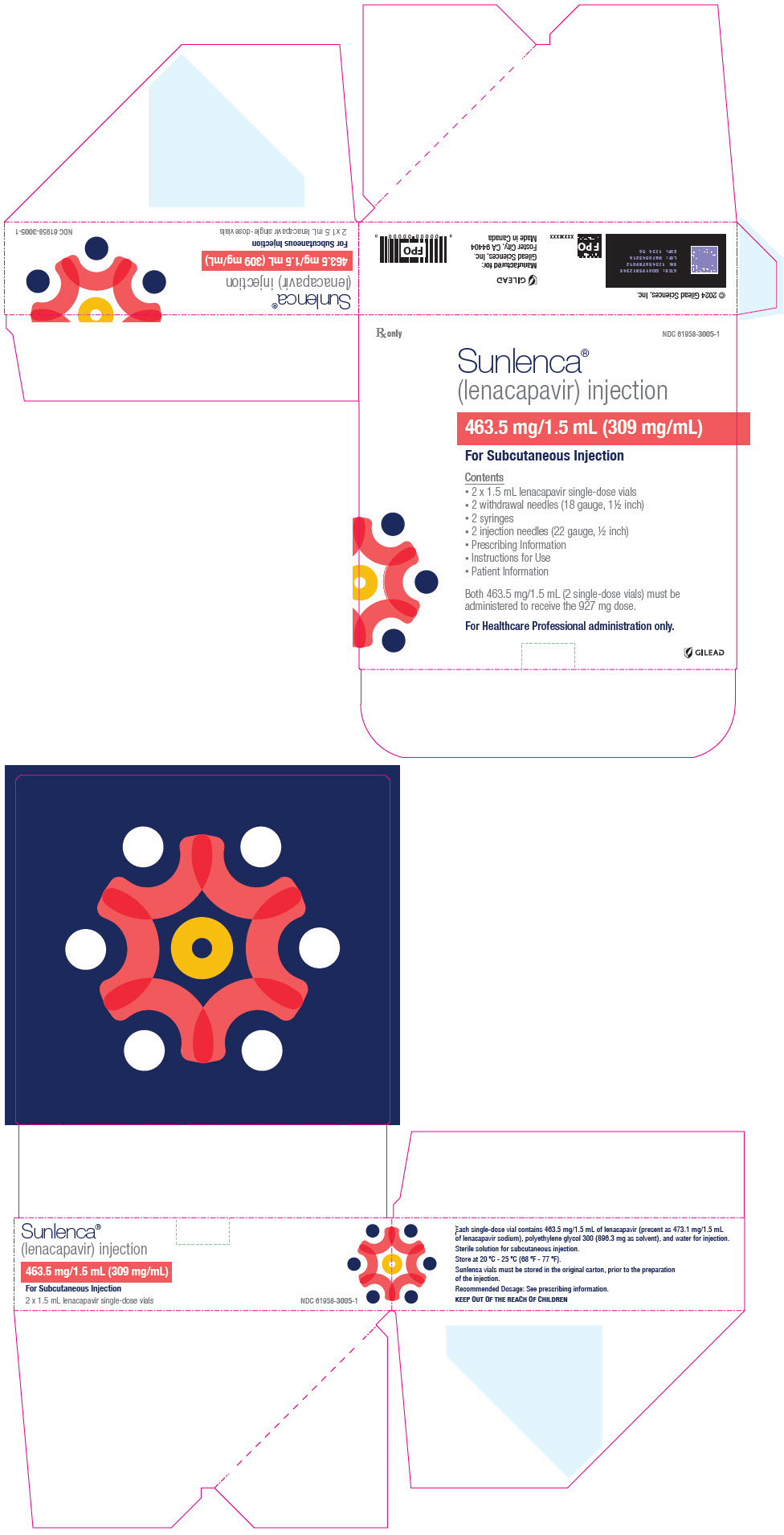
PRINCIPAL DISPLAY PANEL - Kit Carton - 3005Rx only - NDC 61958-3005-1 - Sunlenca® (lenacapavir) injection - 463.5 mg/1.5 mL (309 mg/mL) For Subcutaneous Injection - Contents - 2 x 1.5 mL lenacapavir single-dose vials - 2 withdrawal needles (18 ...
-
INGREDIENTS AND APPEARANCEProduct Information