Label: VORAXAZE- glucarpidase injection, powder, for solution
- NDC Code(s): 50633-210-11
- Packager: BTG International Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated November 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VORAXAZE - ®safely and effectively. See full prescribing information for VORAXAZE - ®. VORAXAZE (glucarpidase) for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEVORAXAZE is indicated to reduce toxic plasma methotrexate concentration (greater than 1 micromole per liter) in adult and pediatric patients with delayed methotrexate clearance (plasma ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - The recommended dosage of VORAXAZE is 50 Units per kilogram (kg) as a single intravenous injection administered over 5 minutes. Flush intravenous line before and after ...
-
3 DOSAGE FORMS AND STRENGTHSFor Injection: 1,000 Units as a white lyophilized powder in a single-dose vial for reconstitution.
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Serious Hypersensitivity Reactions - Serious hypersensitivity reactions occurred in less than 1% of patients [ see Adverse Reactions ( 6.1) ]. 5.2 Interference with ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Serious Hypersensitivity Reactions - [Warnings and Precautions ( 5.1)]. 6.1 ...
-
7 DRUG INTERACTIONS7.1 Effects of VORAXAZE on Leucovorin - VORAXAZE can decrease leucovorin concentration, which may decrease the effect of leucovorin rescue unless leucovorin is dosed as recommended - [see ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on VORAXAZE use in pregnant women or animal reproduction studies to evaluate for a drug-associated risk of major birth defects ...
-
11 DESCRIPTIONGlucarpidase is a carboxypeptidase produced by recombinant DNA technology in genetically modified - Escherichia coli.Glucarpidase is a 390-amino acid homodimer protein with a molecular weight of ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Glucarpidase is a recombinant bacterial enzyme that hydrolyzes the carboxyl- terminal glutamate residue from folic acid and classical antifolates such as ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Glucarpidase has not been evaluated in animals for carcinogenic or mutagenic potential or for impairment of fertility.
-
14 CLINICAL STUDIESThe efficacy of VORAXAZE was evaluated in a subset of 22 patients enrolled in Study 1 (NCT00001298), a single-arm, open-label study in patients who had markedly delayed methotrexate clearance ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGVORAXAZE (glucarpidase) for injection is supplied as a sterile, preservative-free white lyophilized powder in an individually packaged glass single-dose vial closed with a bromo butyl elastomeric ...
-
17 PATIENT COUNSELING INFORMATIONSerious Hypersensitivity Reactions - Inform patients that hypersensitivity reactions, including potentially serious reactions, may occur following a dose of VORAXAZE and to immediately report any ...
-
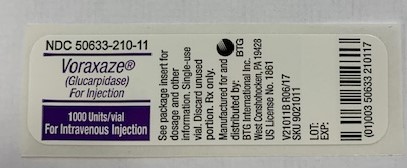
PRINCIPAL DISPLAY PANELPackage Label - Principal Display Panel - VORAXAZE Vial
-
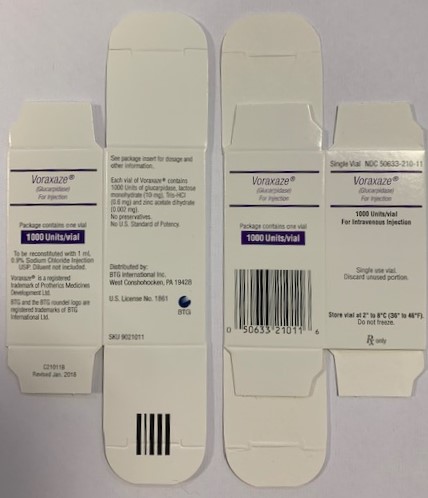
PRINCIPAL DISPLAY PANELPackage Label - Principal Display Panel - VORAXAZE Carton
-
INGREDIENTS AND APPEARANCEProduct Information