Label: VEOZAH- fezolinetant tablet, film coated
- NDC Code(s): 0469-2460-07, 0469-2460-28, 0469-2660-30, 0469-2660-90, view more
- Packager: Astellas Pharma US, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use VEOZAH safely and effectively. See full prescribing information for VEOZAH. VEOZAH® (fezolinetant) tablets, for oral use - Initial ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: RISKS OF HEPATOTOXICITY
Hepatotoxicity has occurred with the use of VEOZAH in the postmarketing setting (5.1).
- •
- Perform hepatic laboratory tests prior to initiation of treatment to evaluate for hepatic function and injury. Do not start VEOZAH if either aminotransferase is ≥ 2 x the upper limit of normal (ULN) or if the total bilirubin is ≥ 2 x ULN for the evaluating laboratory.
- •
- Perform follow-up hepatic laboratory testing monthly for the first 3 months, at 6 months, and 9 months of treatment (2.1, 5.1).
- •
- Advise patients to discontinue VEOZAH immediately and seek medical attention including hepatic laboratory tests if they experience signs or symptoms that may suggest liver injury (new onset fatigue, decreased appetite, nausea, vomiting, pruritus, jaundice, pale feces, dark urine, or abdominal pain) (2.1, 5.1).
- •
- Discontinue VEOZAH if transaminase elevations are > 5 x ULN, or if transaminase elevations are > 3 x ULN and the total bilirubin level is > 2 x ULN.
- •
- If transaminase elevations > 3 x ULN occur, perform more frequent follow-up hepatic laboratory tests until resolution (5.1).
-
1 INDICATIONS AND USAGEVEOZAH® is indicated for the treatment of moderate to severe vasomotor symptoms due to menopause.
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosage - Take a single 45 mg VEOZAH tablet orally once daily with or without food. Take VEOZAH with liquids and swallow whole. Do not cut, crush, or chew tablets. Administer ...
-
3 DOSAGE FORMS AND STRENGTHSTablets: 45 mg, round, light red, film-coated tablets, debossed with the Astellas logo and ‘645’ on the same side.
-
4 CONTRAINDICATIONSVEOZAH is contraindicated in women with any of the following conditions: • Known cirrhosis [see Warnings and Precautions (5.1), Use in Specific Populations (8.7), and Clinical Pharmacology ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hepatotoxicity - In three clinical trials, elevations in serum transaminase [alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST)] levels > 3 x the upper limit of normal ...
-
6 ADVERSE REACTIONS The following serious adverse reactions are discussed elsewhere in the labeling: • Hepatic Transaminase Elevation and Hepatotoxicity [see Warnings and Precautions (5.1)]. 6.1 Clinical Trials ...
-
7 DRUG INTERACTIONS7.1 Effect of Other Drugs on VEOZAH - CYP1A2 Inhibitors - VEOZAH is a substrate of CYP1A2. Concomitant use of VEOZAH with drugs that are weak, moderate, or strong CYP1A2 inhibitors, increase the ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no data on VEOZAH use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal ...
-
10 OVERDOSAGETreatment of overdose consists of discontinuation of VEOZAH therapy with institution of appropriate symptomatic care.
-
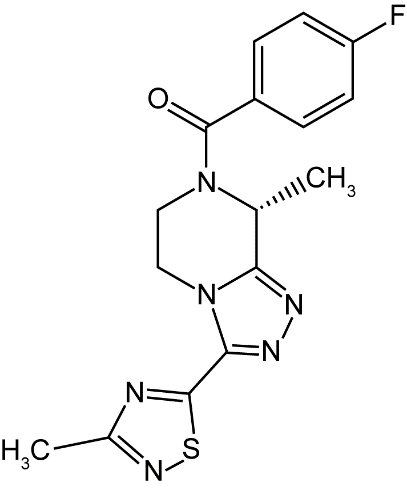
11 DESCRIPTIONVEOZAH (fezolinetant) is a small-molecule NK3 receptor antagonist. The chemical name of fezolinetant is ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - VEOZAH is a neurokinin 3 (NK3) receptor antagonist that blocks neurokinin B (NKB) binding on the kisspeptin/neurokinin B/dynorphin (KNDy) neuron to modulate neuronal ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - In a 2-year female rat carcinogenicity study and a 26-week carcinogenicity study in rasH2 transgenic mice, there was ...
-
14 CLINICAL STUDIES14.1 Effects on Vasomotor Symptoms in Postmenopausal Women - The efficacy of VEOZAH for the treatment of moderate to severe vasomotor symptoms due to menopause was evaluated in the first 12-week ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - VEOZAH (fezolinetant) 45 mg tablets are supplied as round, light red, film-coated tablets, debossed with the Astellas logo and ‘645’ on the same side. VEOZAH tablets are ...
-
17 PATIENT COUNSELING INFORMATIONAdvise patients to read the FDA-approved patient labeling (Patient Information). Evaluation of Hepatic Injury During Treatment with VEOZAH - Inform patients that they will have to have a blood ...
-
PATIENT PACKAGE INSERTPATIENT INFORMATION - VEOZAH® (vee-O-zah) (fezolinetant) tablets, for oral use - What is the most important information I should know about VEOZAH? VEOZAH can cause serious ...
-
Package/Label Display Panel – VEOZAH Bottle Carton Label
NDC 0469-2660-30 - Rx Only - VEOZAH® (fezolinetant) tablets - 45 mg - 30 tablets
-
Package/Label Display Panel – VEOZAH Sample Bottle Carton Label
NDC 0469-2460-07 - PROFESSIONAL SAMPLE-NOT FOR SALE - Rx Only - VEOZAH® (fezolinetant) tablets - 45 mg - 7 tablets
-
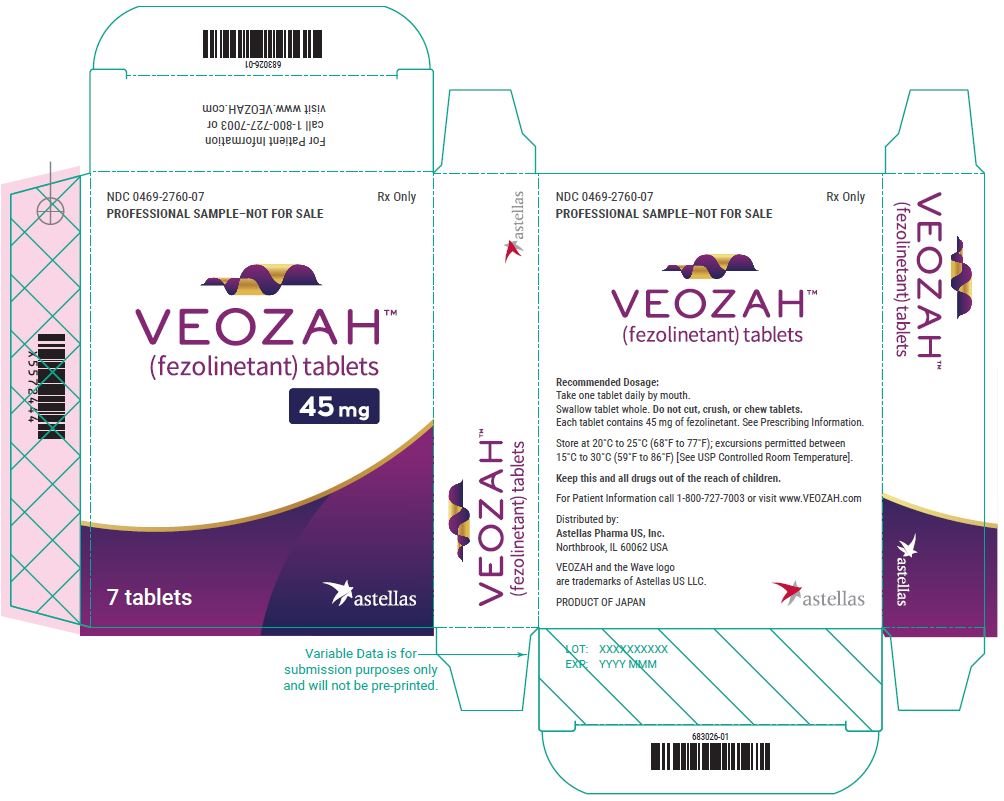
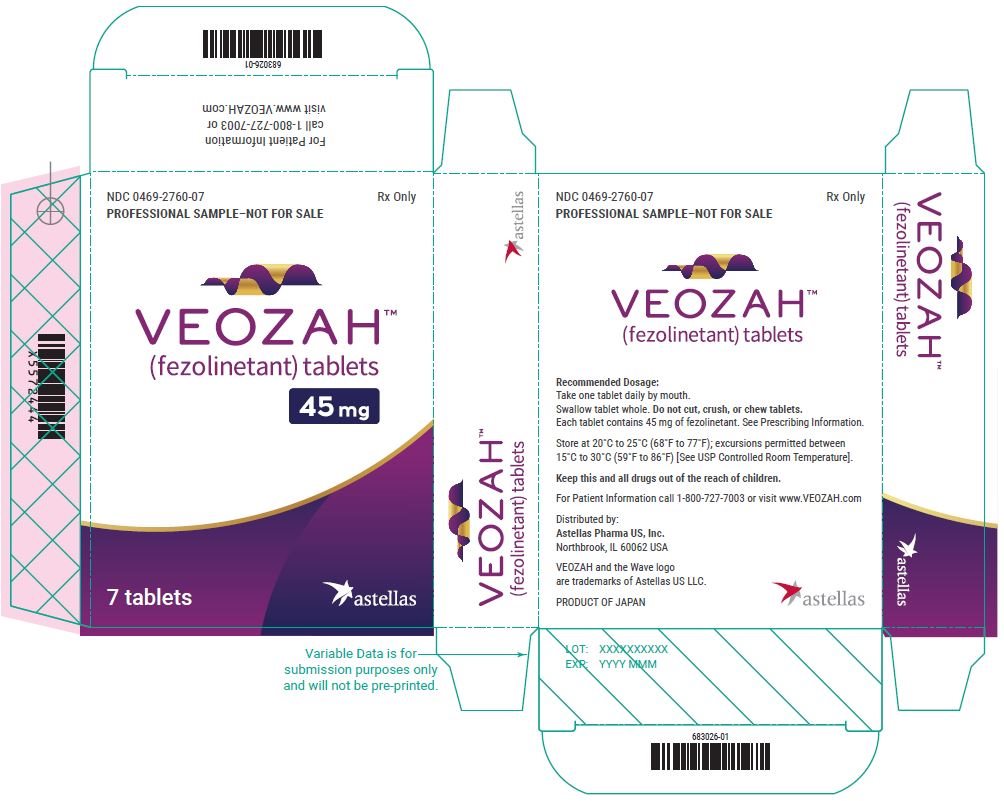
Package/Label Display Panel- VEOZAH Sample Blister Carton Label
NDC 0469-2760-07 - PROFESSIONAL SAMPLE-NOT FOR SALE - Rx Only - VEOZAH® (fezolinetant) tablets - 45 mg - 7 tablets
-
INGREDIENTS AND APPEARANCEProduct Information