Label: LIPIODOL- ethiodized oil injection
- NDC Code(s): 67684-1901-1, 67684-1901-2
- Packager: Guerbet LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated April 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LIPIODOL safely and effectively. See full prescribing information for LIPIODOL.
LIPIODOL® (Ethiodized Oil) Injection, for intrauterine, intralymphatic, or intra-arterial use
Initial U.S. Approval: 1954WARNING: FOR INTRALYMPHATIC, INTRAUTERINE AND SELECTIVE HEPATIC INTRA-ARTERIAL USE ONLY
See Full Prescribing Information for complete Boxed Warning
Pulmonary and cerebral embolism can result from inadvertent intravascular injection or intravasation of Lipiodol. Inject Lipiodol slowly with radiologic monitoring; do not exceed recommended dose (5.1).RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Lipiodol is an oil-based radiopaque contrast agent indicated for: (1)
- hysterosalpingography in adults
- lymphography in adult and pediatric patients
- selective hepatic intra-arterial use for imaging tumors in adults with known hepatocellular carcinoma (HCC)
DOSAGE AND ADMINISTRATION
Use a disposable syringe to draw and inject Lipiodol. (2)
-
Hysterosalpingography
Inject increments of 2 mL of Lipiodol into the endometrial cavity until tubal patency is determined; stop the injection if the patient develops excessive discomfort. Inject with radiologic monitoring. -
Lymphography
Inject Lipiodol into a lymphatic vessel with radiologic monitoring.
Adults: - unilateral lymphography of the upper extremities: 2 to 4 mL
- unilateral lymphography of the lower extremities: 6 to 8 mL
- penile lymphography: 2 to 3 mL
- cervical lymphography: 1 to 2 mL
Pediatric patients: - Inject a minimum of 1 mL to a maximum of 6 mL according to the anatomical area to be visualized. Do not exceed 0.25 mL/kg.
-
Selective Hepatic Intra-arterial Use
Inject 1.5 to 15 mL of Lipiodol slowly under continuous radiologic monitoring. Do not exceed 20 mL total dosage.
DOSAGE FORMS AND STRENGTHS
Each mL of Lipiodol contains 480 mg Iodine organically combined with ethyl esters of fatty acids of poppy seed oil. (3)
CONTRAINDICATIONS
Hypersensitivity to Lipiodol, hyperthyroidism, traumatic injuries, recent hemorrhage or bleeding. (4)
-
Lipiodol Hysterosalpingography is contraindicated in:
pregnancy, acute pelvic inflammatory disease, marked cervical erosion, endocervicitis and intrauterine bleeding, in the immediate pre-or postmenstrual phase, and within 30 days of curettage or conization or patients with known or suspected reproductive tract neoplasia due to the risk of peritoneal spread of neoplasm. -
Lipiodol Lymphography is contraindicated in:
right to left cardiac shunt, advanced pulmonary disease, tissue trauma or hemorrhage, advanced neoplastic disease with expected lymphatic obstruction, previous surgery interrupting the lymphatic system, or radiation therapy to the examined area. -
Lipiodol Selective Hepatic Intra-arterial Injection is contraindicated in:
the presence of dilated bile ducts unless external biliary drainage was performed before injection.
WARNINGS AND PRECAUTIONS
- Pulmonary and cerebral embolism: avoid use in patients with severely impaired lung function, cardiorespiratory failure or right-sided cardiac overload (5.1)
- Hypersensitivity reactions: avoid use in patients with a history of sensitivity to other iodinated contrast agents, bronchial asthma or allergic disorders because of an increased risk of a hypersensitivity reaction to Lipiodol (5.2)
- Exacerbation of chronic liver disease (5.3)
- Thyroid dysfunction (5.4)
ADVERSE REACTIONS
Adverse reactions caused by Lipiodol include hypersensitivity reactions, pulmonary embolism, pulmonary dysfunction, exacerbation of liver disease, procedural complications, abdominal pain, fever, nausea, vomiting, and thyroid dysfunction. (6)
To report SUSPECTED ADVERSE REACTIONS, contact GUERBET LLC at 1-877-729-6679 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch (6)
DRUG INTERACTIONS
Lipiodol may interfere with thyroid function testing and with radioactive iodine uptake by the thyroid tissue during diagnostic or therapeutic procedures. (7.1)
USE IN SPECIFIC POPULATIONS
- Pregnancy: Consider thyroid function testing in pregnant women and their infants if exposed to Lipiodol either before or during pregnancy, if clinically indicated (8.1)
- Lactation: Consider thyroid function testing in a breastfed infant whose mother was exposed to Lipiodol or, if clinically indicated (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Guidelines
2.2 Drug Handling
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Pulmonary and Cerebral Embolism
5.2 Hypersensitivity Reactions
5.3 Exacerbation of Chronic Liver Disease
5.4 Thyroid Dysfunction
6 ADVERSE REACTIONS
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Interference with Iodine-Based Diagnostic Tests and Iodine-Based Radiotherapy
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: FOR INTRALYMPHATIC, INTRAUTERINE AND SELECTIVE HEPATIC INTRA-ARTERIAL USE ONLY
Pulmonary and cerebral embolism can result from inadvertent intravascular injection or intravasation of Lipiodol.
Inject Lipiodol slowly with radiologic monitoring; do not exceed recommended dose (5.1). - 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Guidelines
Draw Lipiodol into a disposable syringe.
Use the smallest possible amount of Lipiodol according to the anatomical area to be visualized.Hysterosalpingography
Using aseptic technique inject Lipiodol into the endometrial cavity with fluoroscopic control. Inject increments of 2 mL of Lipiodol until tubal patency is determined; stop the injection if patient develops excessive discomfort. The total volume to be injected depends on the
volume of the uterine cavity, usually not exceeding 15 mL. A 24-hour image can be obtained if, after slow peritoneal spillage, the evaluation of adhesions is needed. Perform the procedure during the follicular phase of the menstrual cycle.Before using Lipiodol exclude the presence of these conditions: pregnancy, uterine bleeding and endocervicitis, acute pelvic inflammatory disease, the immediate pre-or postmenstrual phase or within 30 days of curettage or conization. [see Contraindications (4) & Pregnancy (8.1)].
Lymphography
Inject Lipiodol into a lymphatic vessel under radiologic guidance to prevent inadvertent venous administration or intravasation.
Adults:
- unilateral lymphography of the upper extremities 2 to 4 mL
- unilateral lymphography of the lower extremities 6 to 8 mL
- penile lymphography 2 to 3 mL
- cervical lymphography 1 to 2 mL
Pediatric patients:
- Inject a minimum of 1 mL to a maximum of 6 mL according to the anatomical area to be visualized. Do not exceed 0.25 mL/kg.
The following method is recommended for lymphography of the upper or lower extremities. Start the injection of Lipiodol into a lymphatic channel at a rate not to exceed 0.2 mL per minute. Inject the total dose of Lipiodol in no less than 1.25 hours. Use frequent radiologic monitoring to determine the appropriate injection rate and to follow the progress of Lipiodol within the lymphatics. Interrupt the injection if the patient experiences pain. Terminate the injection if lymphatic blockage is present to minimize introduction of Lipiodol into the venous circulation via lymphovenous channels. Terminate the injection as soon as Lipiodol is radiographically evident in the thoracic duct to minimize entry of Lipiodol into the subclavian vein and pulmonary embolization. Obtain immediate post-injection images. Re-image at 24 or 48 hours to evaluate nodal architecture.
Selective Hepatic Intra-arterial Injection
Determine the dose depending on the tumor size, local blood flow in the liver and in the tumor(s).
- Inject from 1.5 to 15 mL slowly under continuous radiologic monitoring. Stop the injection when stagnation or reflux is evident. Limit the dose to only the quantity required for adequate visualization. The total dose of Lipiodol administered should not exceed 20 mL.
2.2 Drug Handling
Inspect Lipiodol visually for particulate matter and discoloration before administration. Do not use the solution if particulate matter is present or if the container appears damaged. Lipiodol is a clear, pale yellow to amber colored oil; do not use if the color has darkened.
Draw Lipiodol into a disposable syringe and use promptly. Discard any unused portion of Lipiodol.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Lipiodol is contraindicated in patients with hypersensitivity to Lipiodol, hyperthyroidism, traumatic injuries, recent hemorrhage or bleeding.
Hysterosalpingography
Lipiodol hysterosalpingography is contraindicated in pregnancy, acute pelvic inflammatory disease, marked cervical erosion, endocervicitis and intrauterine bleeding, in the immediate pre-or postmenstrual phase, and within 30 days of curettage or conization or patients with known or suspected reproductive tract neoplasia due to the risk of peritoneal spread of neoplasm. [see Use in Specific Populations (8.1).]
Lymphography
Lipiodol Lymphography is contraindicated in patients with a right to left cardiac shunt, advanced pulmonary disease, tissue trauma or hemorrhage advanced neoplastic disease with expected lymphatic obstruction, previous surgery interrupting the lymphatic system, radiation therapy to the examined area.
Selective Hepatic Intra-arterial Use Patients with HCC
Lipiodol use is contraindicated in areas of the liver where the bile ducts are dilated unless external biliary drainage was performed before injection.
-
5 WARNINGS AND PRECAUTIONS
5.1 Pulmonary and Cerebral Embolism
Pulmonary embolism may occur immediately or after a few hours to days from inadvertent systemic vascular injection or intravasation of Lipiodol and cause decreased pulmonary diffusing capacity and pulmonary blood flow, pulmonary infarction, acute respiratory distress syndrome and fatalities. Embolization of Lipiodol to brain and other major organs may occur. Avoid use of Lipiodol in patients with severely impaired lung function, cardiorespiratory failure, or right–sided cardiac overload. Perform radiological monitoring during the Lipiodol injection. Do not exceed the recommended maximum dose and rate of injection of Lipiodol. During lymphography to minimize the risk of pulmonary embolism obtain radiographic confirmation of intralymphatic (rather than venous) injection, and terminate the procedure when Lipiodol becomes visible in the thoracic duct or lymphatic obstruction is observed.
5.2 Hypersensitivity Reactions
Anaphylactoid and anaphylactic reactions with cardiovascular, respiratory or cutaneous manifestations, ranging from mild to severe, including death, have uncommonly occurred following Lipiodol administration. Avoid use in patients with a history of sensitivity to other iodinated contrast agents, bronchial asthma or allergic disorders because of an increased risk of a hypersensitivity reaction to Lipiodol. Administer Lipiodol only in situations where trained personnel and therapies are promptly available for the treatment of hypersensitivity reactions, including personnel trained in resuscitation; ensure continuous medical monitoring and maintain an intravenous access line. Most hypersensitivity reactions to Lipiodol occur within half an hour after administration. Delayed reactions can occur up to several days after administration. Observe patients for signs and symptoms of hypersensitivity reactions during and for at least 30 minutes following Lipiodol administration.
5.3 Exacerbation of Chronic Liver Disease
Lipiodol hepatic intra-arterial administration can exacerbate the following conditions: portal hypertension and cause variceal bleeds due to obstruction of the intrahepatic portal channels by opening a pre sinusoidal anastomosis; hepatic ischemia and cause liver enzyme elevations, fever and abdominal pain; hepatic failure and cause ascites and encephalopathy. Hepatic vein thrombosis, irreversible liver insufficiency and fatalities have been reported. Procedural risks include vascular complications and infections.
5.4 Thyroid Dysfunction
Iodinated contrast media can affect thyroid function because of the iodide content and can cause hyperthyroidism or hypothyroidism. Ethiodized oil may remain in the body for several months, depending on dose administered and route of administration. Keep the dose of Lipiodol as low as possible and consider monitoring thyroid function closely for several months after administration of Lipiodol.
-
6 ADVERSE REACTIONS
6.2 Postmarketing Experience
The following adverse reactions (Table 1) have been identified during post approval use of Lipiodol. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following adverse reactions are described in more detail in other sections of the prescribing information:
Pulmonary and cerebral embolism [see Warnings and Precautions (5.1)]
Hypersensitivity reactions [see Warnings and Precautions (5.2)]
Exacerbation of chronic liver disease [see Warnings and Precautions (5.3)]Thyroid dysfunction [see Warnings and Precautions (5.4)]. Thyroid dysfunction in the mother and the infant of a subsequent pregnancy
[See Use in Specific Populations (8.1)]Table 1: Adverse Reactions in the Postmarketing Experience System Organ Class
Adverse Reaction
Eye disorders
retinal vein thrombosis
General disorders and administration site conditions
fever, pain, granuloma
Immune system disorders
hypersensitivity, anaphylactic reaction, anaphylactoid reaction, cardiovascular reactions
Nervous system disorders
cerebral embolism
Respiratory, thoracic and mediastinal disorders
pulmonary embolism, dyspnea, cough, acute respiratory distress syndrome
Urinary system disorders
renal insufficiency
Hysterosalpingography
Abdominal pain, foreign body reactions, exacerbation of pelvic inflammatory disease, salpingitis or pelvic peritonitis have been reported
after the examination in case of latent infection.Lymphography
Lymphangitis, thrombophlebitis, edema or exacerbation of preexisting lymphedema, dyspnea and cough, iodism (headache, soreness of mouth and pharynx, coryza and skin rash), allergic dermatitis, lipogranuloma, delayed healing at the site of incision.
Selective Hepatic Intra-arterial Injection
Abdominal pain, nausea, and vomiting are the most common reactions; other reactions include hepatic vein thrombosis, hepatic ischemia, liver enzymes abnormalities, transitory decrease in liver function, liver decompensation and renal insufficiency. Procedural risks include vascular complications and infections.
-
7 DRUG INTERACTIONS
7.1 Interference with Iodine-Based Diagnostic Tests and Iodine-Based Radiotherapy
Following Lipiodol administration, ethiodized oil remains in the body for several months. Ethiodized oil interferes with radioactive iodine uptake by the thyroid for several weeks to months may impair visualization of thyroid scintigraphy and reduce effectiveness of iodine 131 treatment.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Hysterosalpingography is contraindicated in pregnant women due to the potential risk to the fetus from an intrauterine procedure [see
Contraindications (4)]. The use of Lipiodol before or during pregnancy may interfere with thyroid function in both the pregnant woman
and her fetus and may affect fetal development. There are maternal, fetal, and neonatal clinical considerations for women who are exposed to Lipiodol either before or during pregnancy (see Clinical Considerations). Rare pregnancy outcomes reported in the post marketing setting with Lipiodol use in pregnant women are insufficient to evaluate for a drug-associated risk of major birth defects or miscarriage (see Data). Animal reproduction studies have not been conducted using the indicated routes of administration of Lipiodol, it was not embryotoxic or teratogenic in animal studies with oral administration.The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a
background risk of major birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of
major birth defects and miscarriages in clinically recognized pregnancies is 2-4% and 15-20%, respectively.Clinical Considerations
Maternal adverse reactions
The use of Lipiodol before or during pregnancy may interfere with the thyroid function of the pregnant woman. Untreated hypothyroidism in pregnancy is associated with adverse perinatal outcomes, such as spontaneous abortion, preeclampsia, preterm birth, abruptio placentae, and fetal death. Consider thyroid function testing during pregnancy if a woman was exposed to Lipiodol either before or during pregnancy or if clinically indicated [see Warnings and Precautions (5.4)].Fetal / Neonatal adverse reactions
The use of Lipiodol before or during pregnancy causes iodide transfer across the placenta which may interfere with fetal thyroid function
and may affect fetal development. Untreated hypothyroidism is associated with increased fetal risk of low birth weight, fetal distress, and
impaired neuropsychological development. Consider thyroid function testing in infants whose mothers were exposed to Lipiodol before
and during pregnancy or if clinically indicated [see Warnings and Precautions (5.4)].Data
Human Data
In a prospective study, twenty-two euthyroid women with infertility underwent hysterosalpingography, and thyroid stimulating hormone,
serum iodine concentration, and urinary iodide/creatine excretion were evaluated at 4, 8, 12, 24 weeks. Six patients were followed upto12 months. The median value of urinary iodide excretion peaked at four weeks post-procedure and remained elevated at 8, 12, 24 weeks post-procedure compared to pre-procedure. Three patients showed elevated TSH (5 uIU/L) with normal T4 at 4 or 8 weeks after
hysterosalpingography.In addition, there are post marketing reports of goiters and hypothyroidism in fetuses and infants whose mothers were exposed to Lipiodol for hysterosalpingography prior to pregnancy.
Animal Data
Lipiodol was not embryotoxic or teratogenic in rats after oral administration of up to 250 mg Iodine/kg each day between gestation days 6 to 17, or in rabbits after 4-5 intermittent (once every three days) oral administrations of 12.5 mg Iodine/kg between gestation days 6 to 18. Maternal
toxicity was observed in rats at 250 mg Iodine/kg/day.8.2 Lactation
Risk Summary
Ethiodized oil is present in human milk.
There are no data on the effects of ethiodized oil on the breastfed infant, or effects on milk production, however, the use of Lipiodol may
increase the concentration of iodide in human milk and may interfere with the thyroid function of the breastfed infant. Consider thyroid
function testing in a breastfed infant whose mother was exposed to Lipiodol or if clinically indicated. The developmental and health
benefits of breastfeeding should be considered along with the mother’s clinical need for Lipiodol and any potential adverse effects on the
breastfed child from Lipiodol or from the underlying maternal condition.8.3 Females and Males of Reproductive potential
Pregnancy Testing
Confirm that the patient has a negative pregnancy test within 24 hours before Lipiodol administration for hysterosalpingography [see
Dosage and Administration (2.1), Contraindications (4)]. - 10 OVERDOSAGE
-
11 DESCRIPTION
Lipiodol, ethiodized oil injection, is a sterile injectable radio-opaque agent. Each milliliter contains 480 mg of Iodine organically combined with ethyl esters of fatty acids of poppy seed oil. The precise structure of Lipiodol is unknown.
Lipiodol is a sterile, clear, pale yellow to amber colored oil. Lipiodol has a viscosity of 34 – 70 mPa·s at 20°C, and a density of 1.28 g/cm3 at 20°C.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Lipiodol opacifies vessels and body structures in the path of the flow of the contrast medium, permitting visualization of the internal structures.
12.2 Pharmacodynamics
Following administration of Lipiodol, the degree of enhancement is directly related to the local iodine content in the body structures to be imaged.
12.3 Pharmacokinetics
Following intra-arterial administration of Lipiodol, ethiodized oil retained in normal hepatic parenchyma is phagocytized by the Kupffer cells of the liver and washed out via the hepatic lymphatic system in about 2 to 4 weeks. In HCC, retention in the liver tumor is prolonged, allowing re-imaging of the tumor for four weeks or longer.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenic potential. Lipiodol did not demonstrate mutagenic potential in bacterial reverse mutation assays (in vitro), in a chromosomal aberration test in the mouse lymphoma assay (in vitro), and was negative in an in vivo micronucleus test in rats after intravenous injection of 479 mg I/kg.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
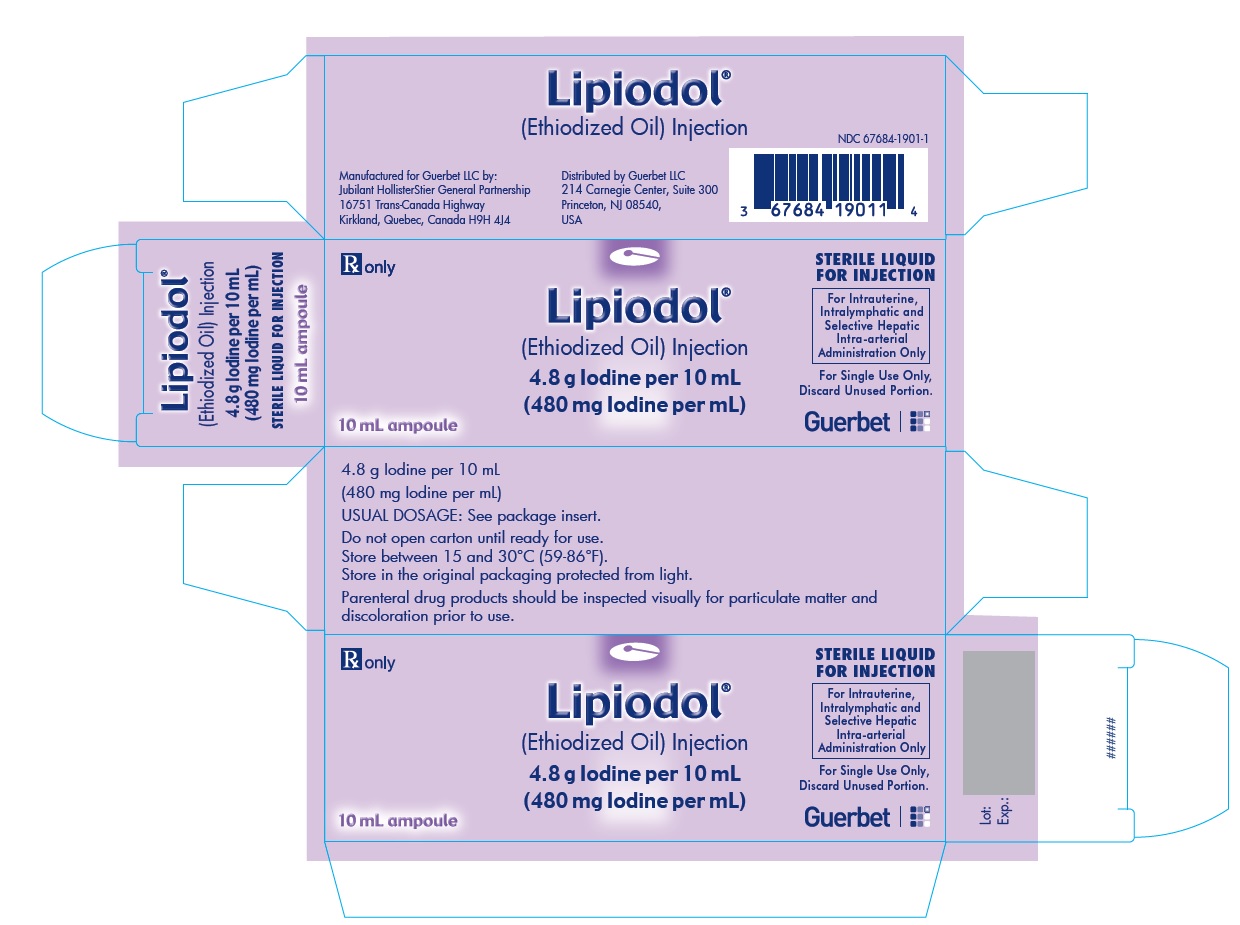
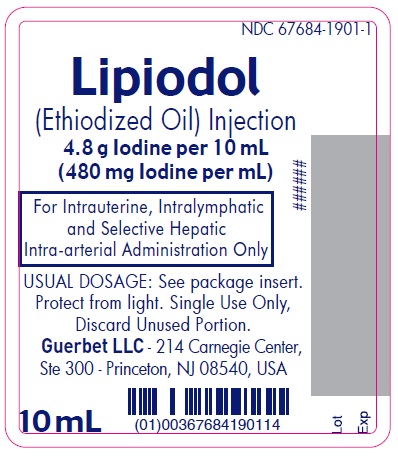
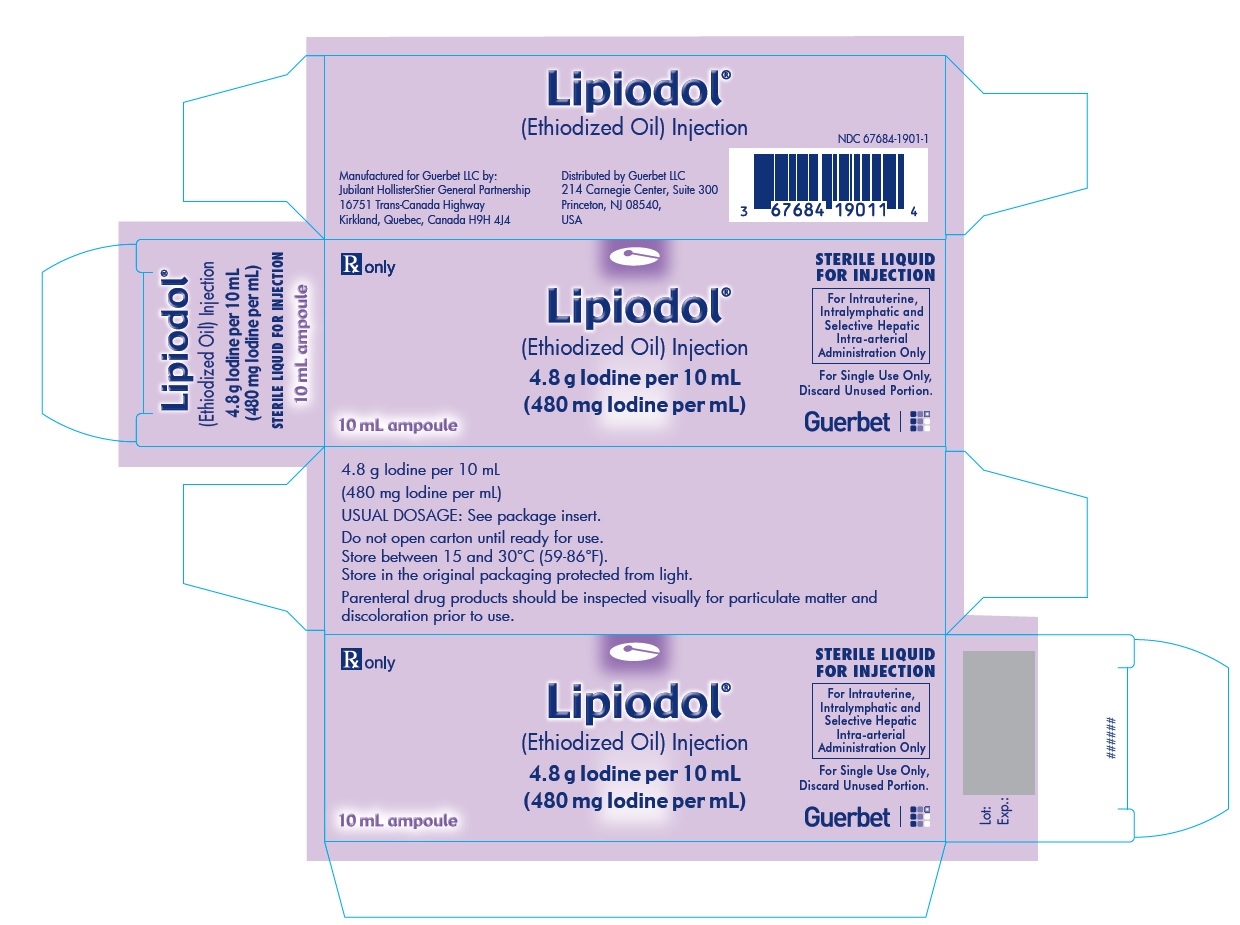
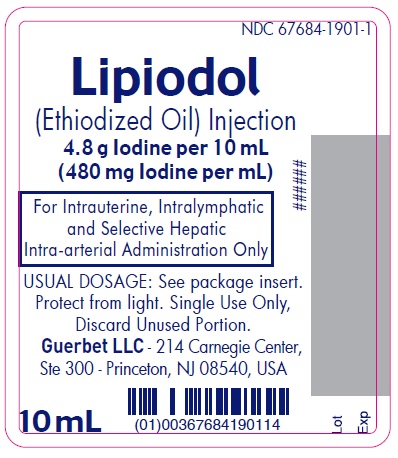
Lipiodol is supplied in a box of one 10 mL ampoule, NDC 67684-1901-1.
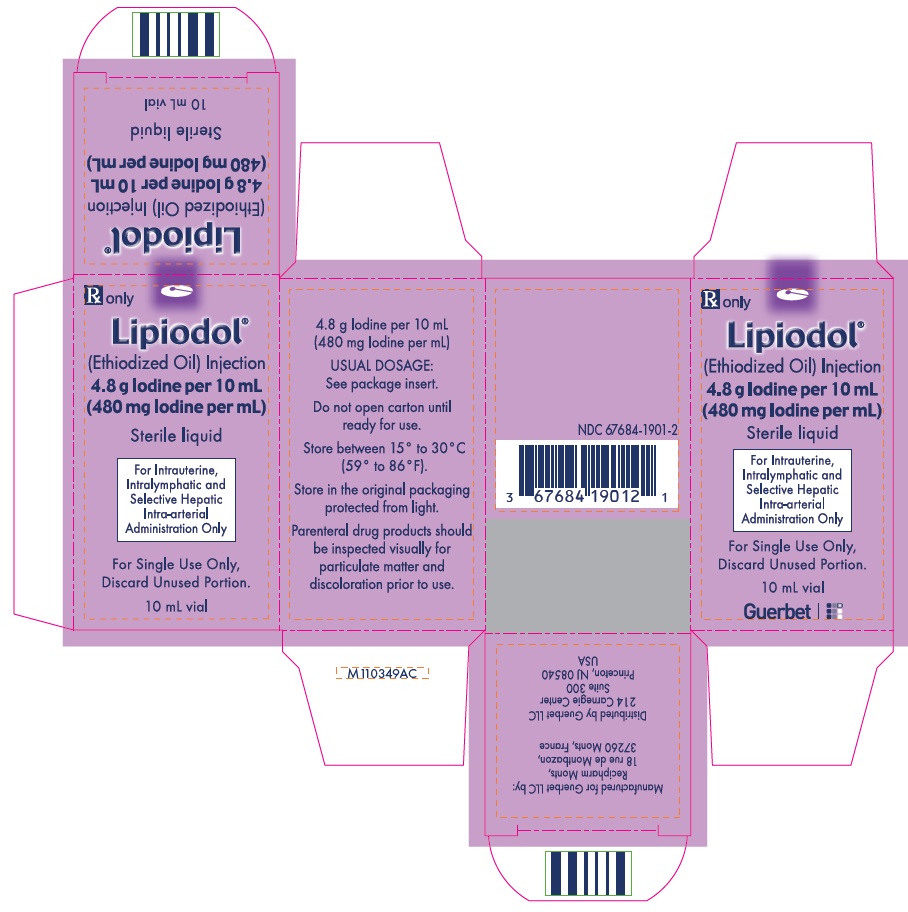
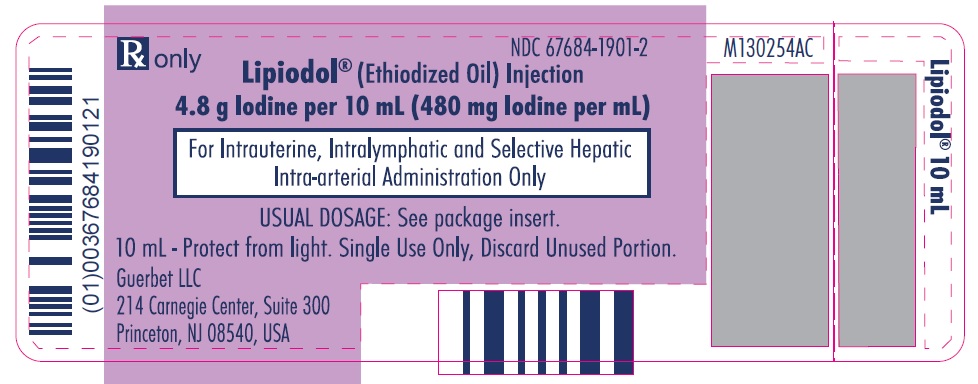
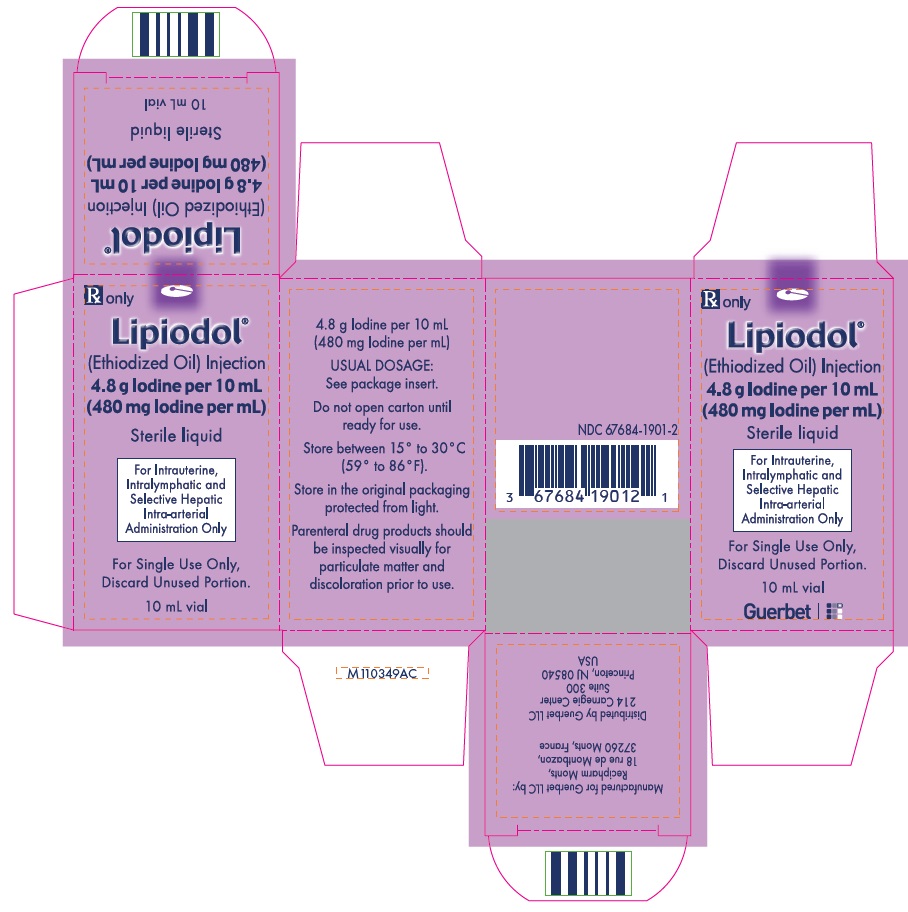
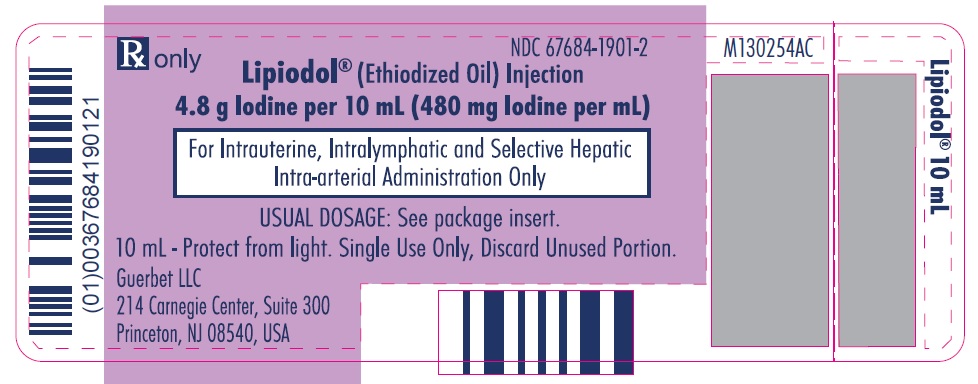
Lipiodol is supplied in a box of one 10 mL vial, NDC 67684-1901-2.
Each vial is closed with a rubber stopper and sealed with an aluminum cap.Store at controlled room temperature 15°-30°C (59°-86°F) [see USP, Controlled Room Temperature (CRT)]. Protect from light. Remove from carton only upon use.
-
17 PATIENT COUNSELING INFORMATION
Hypersensitivity Reactions
Advise the patient concerning the risk of hypersensitivity reactions that can occur both during and after Lipiodol administration. Advise
the patient to report any signs or symptoms of hypersensitivity reactions during the procedure and to seek medical attention for signs or
symptoms experienced after discharge [see Warnings and Precautions (5.2)].Thyroid Dysfunction
Advise patients concerning the risk of hyper- or hypothyroidism flowing the use of Lipiodol due to ethiodized oil remaining in the body
for several months. Instruct patients to follow up with their physician if they become pregnant or develop any signs of thyroid dysfunction in the months after administration [see Warnings and Precautions (5.4), Use in Specific Populations (8.1 and 8.3)]. - SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LIPIODOL
ethiodized oil injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:67684-1901 Route of Administration INTRALYMPHATIC, INTRAUTERINE, INTRA-ARTERIAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ETHIODIZED OIL (UNII: KZW0R0686Q) (IODINE - UNII:9679TC07X4) IODINE 480 mg in 1 mL Inactive Ingredients Ingredient Name Strength POPPY SEED OIL (UNII: 9G3Z76ES9J) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67684-1901-1 1 in 1 CARTON 03/21/2014 1 10 mL in 1 AMPULE; Type 0: Not a Combination Product 2 NDC:67684-1901-2 1 in 1 CARTON 09/28/2018 2 10 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA009190 03/21/2014 Labeler - Guerbet LLC (037876096) Registrant - Guerbet LLC (037876096) Establishment Name Address ID/FEI Business Operations Simafex 381108273 api manufacture(67684-1901) Establishment Name Address ID/FEI Business Operations Jubilant HollisterStier General Partnership 246762764 manufacture(67684-1901) , pack(67684-1901) Establishment Name Address ID/FEI Business Operations RECIPHARM MONTS 777990813 manufacture(67684-1901) , pack(67684-1901) Establishment Name Address ID/FEI Business Operations IFTS 270199391 analysis(67684-1901)