Label: PARSABIV- etelcalcetide injection, solution
- NDC Code(s): 55513-740-01, 55513-740-10, 55513-741-01, 55513-741-10, view more
- Packager: Amgen Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 17, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use PARSABIV safely and effectively. See full prescribing information for PARSABIV. PARSABIV® (etelcalcetide) injection, for ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEPARSABIV is indicated for the treatment of secondary hyperparathyroidism (HPT) in adult patients with chronic kidney disease (CKD) on hemodialysis. Limitations of Use: PARSABIV has not been ...
-
2 DOSAGE AND ADMINISTRATION2.1 Recommended Dosing - Ensure corrected serum calcium is at or above the lower limit of normal prior to PARSABIV initiation, a PARSABIV dose increase, or re-initiation of PARSABIV therapy ...
-
3 DOSAGE FORMS AND STRENGTHSPARSABIV is a single-dose, clear, and colorless solution available as follows: Injection: 2.5 mg/0.5 mL solution in a single-dose vial - Injection: 5 mg/mL solution in a single-dose vial - Injection ...
-
4 CONTRAINDICATIONSHypersensitivity - PARSABIV is contraindicated in patients with known hypersensitivity to etelcalcetide or any of its excipients. Hypersensitivity reactions, including face edema and ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hypocalcemia - PARSABIV lowers serum calcium [see Adverse Reactions (6.1)] and can lead to hypocalcemia, sometimes severe. Significant lowering of serum calcium can cause paresthesias ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections of the labeling: Hypocalcemia [see Warnings and Precautions (5.1)] Worsening Heart Failure [see Warnings and ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data on the use of PARSABIV in pregnant women. In animal reproduction studies, effects were seen at doses associated with maternal ...
-
10 OVERDOSAGEThere is no clinical experience with PARSABIV overdosage. Overdosage of PARSABIV may lead to hypocalcemia with or without clinical symptoms and may require treatment. Although PARSABIV is cleared ...
-
11 DESCRIPTIONPARSABIV (etelcalcetide) is a synthetic peptide calcium-sensing receptor agonist. Etelcalcetide is a white to off-white powder with a molecular formula of C38H73N21O10S2∙xHCl (4 ≤ x ≤ 5) and a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Etelcalcetide is a calcimimetic agent that allosterically modulates the calcium-sensing receptor (CaSR). Etelcalcetide binds to the CaSR and enhances activation of the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No drug-related tumors were observed in Tg rasH2 transgenic mice when etelcalcetide was administered at doses of 0.3, 1, and 3 mg/kg in ...
-
14 CLINICAL STUDIESThe efficacy and safety of PARSABIV for the treatment of secondary hyperparathyroidism in patients with chronic kidney disease receiving hemodialysis three times per week were evaluated in two ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGPARSABIV (etelcalcetide) injection is supplied in a single-dose vial (type I glass) with stopper (fluoropolymer laminated elastomeric) and an aluminum seal with flip-off dust cover containing 5 ...
-
17 PATIENT COUNSELING INFORMATIONHypocalcemia - Advise patients to report symptoms of hypocalcemia, including paresthesias, myalgias, muscle spasms, and seizures, to their healthcare provider [see Warnings and Precautions ...
-
SPL UNCLASSIFIED SECTIONPARSABIV® (etelcalcetide) Manufactured for: KAI Pharmaceuticals, Inc., a wholly owned subsidiary of Amgen, Inc. One Amgen Center Drive - Thousand Oaks, California 91320-1799 - Patent ...
-
PRINCIPAL DISPLAY PANEL - 2.5 mg/0.5 mL Vial Carton10 x 0.5 mL Single-dose Vials - NDC 55513-740-10 - AMGEN® Parsabiv® (etelcalcetide) Injection - 2.5 mg/ 0.5 mL - For Intravenous Use after Hemodialysis - Single-dose vial. Discard unused portion - Store at ...
-
PRINCIPAL DISPLAY PANEL - 5 mg/mL Vial Carton10 x 1 mL Single-dose Vials - NDC 55513-741-10 - AMGEN® Parsabiv® (etelcalcetide) Injection - 5 - mg/mL - For Intravenous Use after Hemodialysis - Single-dose vial. Discard unused portion - Store at 2°C to ...
-
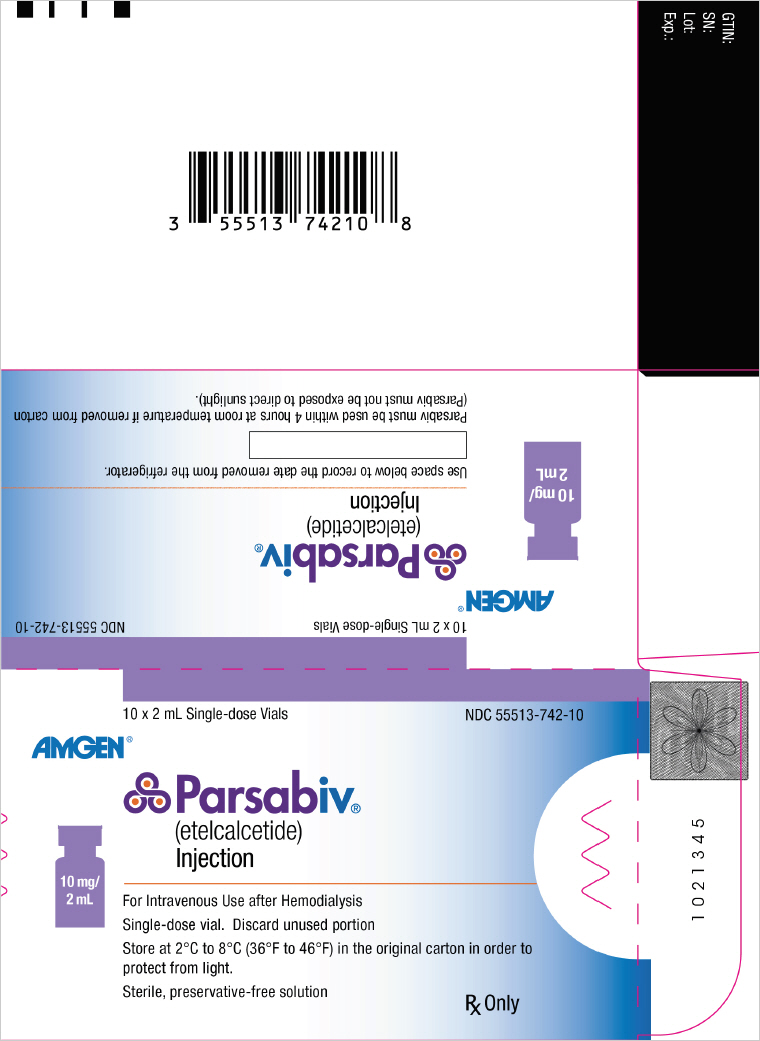
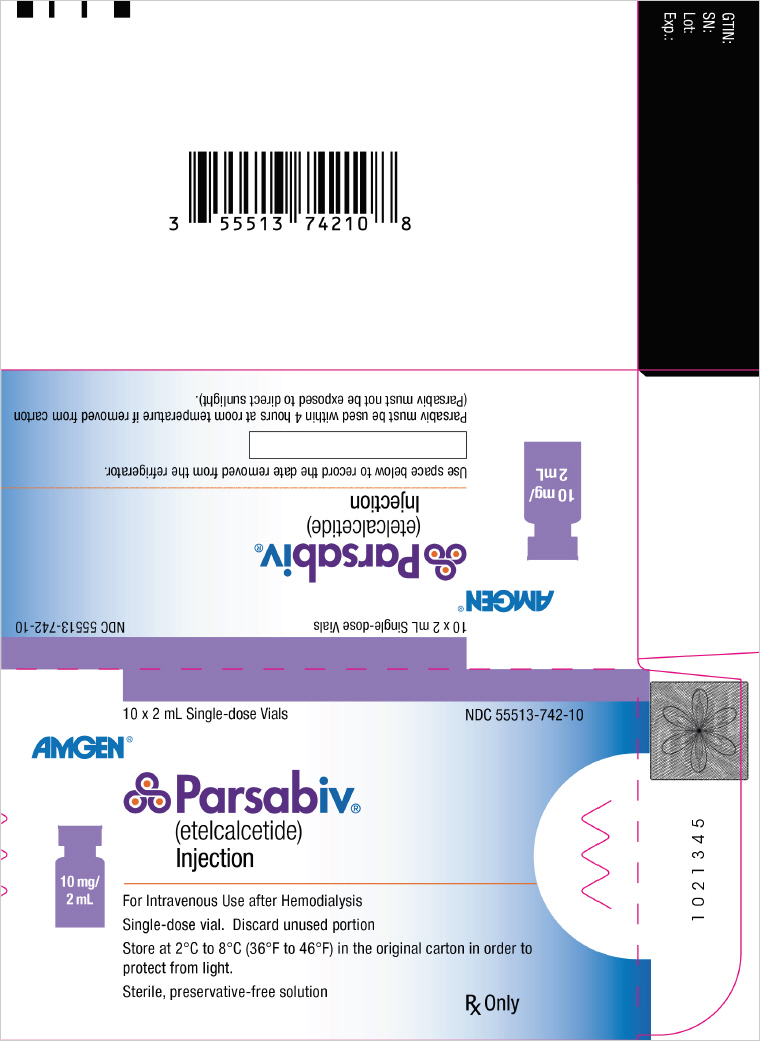
PRINCIPAL DISPLAY PANEL - 10 mg/2 mL Vial Carton10 x 2 mL Single-dose Vials - NDC 55513-742-10 - AMGEN® Parsabiv® (etelcalcetide) Injection - 10 mg/ 2 mL - For Intravenous Use after Hemodialysis - Single-dose vial. Discard unused portion - Store at 2°C ...
-
INGREDIENTS AND APPEARANCEProduct Information