Label: SAVAYSA- edoxaban tosylate tablet, film coated
-
NDC Code(s):
65597-201-07,
65597-201-10,
65597-201-30,
65597-201-50, view more65597-201-70, 65597-201-90, 65597-202-05, 65597-202-07, 65597-202-10, 65597-202-30, 65597-202-50, 65597-202-70, 65597-202-90, 65597-203-05, 65597-203-07, 65597-203-10, 65597-203-30, 65597-203-50, 65597-203-70, 65597-203-90
- Packager: Daiichi Sankyo Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SAVAYSA safely and effectively. See full prescribing information for SAVAYSA. SAVAYSA® (edoxaban) tablets, for oral use - Initial ...These highlights do not include all the information needed to use SAVAYSA safely and effectively. See full prescribing information for SAVAYSA.
SAVAYSA® (edoxaban) tablets, for oral use
Initial U.S. Approval: 2015WARNING: (A) REDUCED EFFICACY IN NONVALVULAR ATRIAL FIBRILLATION PATIENTS WITH CREATININE CLEARANCE (CRCL) > 95 ML/MIN (B) PREMATURE DISCONTINUATION OF SAVAYSA INCREASES THE RISK OF ISCHEMIC EVENTS (C) SPINAL/EPIDURAL HEMATOMA
See full prescribing information for complete boxed warning.
(A) REDUCED EFFICACY IN NONVALVULAR ATRIAL FIBRILLATION PATIENTS WITH CRCL > 95 ML/MIN: SAVAYSA should not be used in patients with CrCL > 95 mL/min. In the ENGAGE AF-TIMI 48 study, nonvalvular atrial fibrillation patients with CrCL > 95 mL/min had an increased rate of ischemic stroke with SAVAYSA 60 mg once daily compared to patients treated with warfarin. In these patients another anticoagulant should be used (5.1).
(B) PREMATURE DISCONTINUATION OF SAVAYSA INCREASES THE RISK OF ISCHEMIC EVENTS: Premature discontinuation of any oral anticoagulant in the absence of adequate alternative anticoagulation increases the risk of ischemic events. If SAVAYSA is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant as described in the transition guidance (2.4, 5.2, 14).
(C) SPINAL/EPIDURAL HEMATOMA: Epidural or spinal hematomas may occur in patients treated with SAVAYSA who are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures (5.4).
INDICATIONS AND USAGE
SAVAYSA is a factor Xa inhibitor indicated:
To reduce the risk of stroke and systemic embolism (SE) in patients with nonvalvular atrial fibrillation (NVAF) (1.1)
-
Limitation of Use for NVAF
SAVAYSA should not be used in patients with creatinine clearance (CrCL) > 95 mL/min because of increased risk of ischemic stroke compared to warfarin at the highest dose studied (60 mg) (1.1)
SAVAYSA is indicated for the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) following 5 to 10 days of initial therapy with a parenteral anticoagulant (1.2)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Tablets: 60 mg, 30 mg, and 15 mg (3)
CONTRAINDICATIONS
- Active pathological bleeding (4)
WARNINGS AND PRECAUTIONS
- Bleeding: Serious and potentially fatal bleeding. Promptly evaluate signs and symptoms of blood loss (5.3)
- Mechanical Heart Valves or Moderate to Severe Mitral Stenosis: Use is not recommended (5.5)
- Increased Risk of Thrombosis in Patients with Triple Positive Antiphospholipid Syndrome: SAVAYSA use not recommended. (5.6)
ADVERSE REACTIONS
Treatment of NVAF: The most common adverse reactions (≥ 5%) are bleeding and anemia (6.1)
Treatment of DVT and PE: The most common adverse reactions (≥ 1%) are bleeding, rash, abnormal liver function tests and anemia (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Daiichi Sankyo, Inc. at 1-877-437-7763 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 10/2023
Close -
Limitation of Use for NVAF
-
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: (A) REDUCED EFFICACY IN NONVALVULAR ATRIAL FIBRILLATION PATIENTS WITH CREATININE CLEARANCE (CRCL) > 95 ML/MIN (B) PREMATURE DISCONTINUATION OF SAVAYSA INCREASES THE RISK OF ISCHEMIC EVENTS (C) SPINAL/EPIDURAL HEMATOMA
1 INDICATIONS AND USAGE
1.1 Reduction in the Risk of Stroke and Systemic Embolism in Nonvalvular Atrial Fibrillation
1.2 Treatment of Deep Vein Thrombosis and Pulmonary Embolism
2 DOSAGE AND ADMINISTRATION
2.1 Nonvalvular Atrial Fibrillation
2.2 Treatment of Deep Vein Thrombosis and Pulmonary Embolism
2.3 Administration Information
2.4 Transition to or from SAVAYSA
2.5 Discontinuation for Surgery and Other Interventions
2.6 Administration Options
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Reduced Efficacy in Nonvalvular Atrial Fibrillation Patients with CrCL > 95 mL/min
5.2 Increased Risk of Stroke with Discontinuation of SAVAYSA in Patients with Nonvalvular Atrial Fibrillation
5.3 Risk of Bleeding
5.4 Spinal/Epidural Anesthesia or Puncture
5.5 Patients with Mechanical Heart Valves or Moderate to Severe Mitral Stenosis
5.6 Increased Risk of Thrombosis in Patients with Triple Positive Antiphospholipid Syndrome
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Anticoagulants, Antiplatelets, Thrombolytics, and SSRIs/SNRIs
7.2 P-gp Inducers
7.3 P-gp Inhibitors
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
8.8 Low Body Weight Consideration for Patients Treated for DVT and/or PE
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Nonvalvular Atrial Fibrillation
14.2 Treatment of Deep Vein Thrombosis and Pulmonary Embolism
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)A. REDUCED EFFICACY IN NONVALVULAR ATRIAL FIBRILLATION PATIENTS WITH CRCL > 95 ML/MIN - SAVAYSA should not be used in patients with CrCL > 95 mL/min. In the ENGAGE AF-TIMI 48 study, nonvalvular ...
WARNING: (A) REDUCED EFFICACY IN NONVALVULAR ATRIAL FIBRILLATION PATIENTS WITH CREATININE CLEARANCE (CRCL) > 95 ML/MIN (B) PREMATURE DISCONTINUATION OF SAVAYSA INCREASES THE RISK OF ISCHEMIC EVENTS (C) SPINAL/EPIDURAL HEMATOMA
A. REDUCED EFFICACY IN NONVALVULAR ATRIAL FIBRILLATION PATIENTS WITH CRCL > 95 ML/MIN
SAVAYSA should not be used in patients with CrCL > 95 mL/min. In the ENGAGE AF-TIMI 48 study, nonvalvular atrial fibrillation patients with CrCL > 95 mL/min had an increased rate of ischemic stroke with SAVAYSA 60 mg once daily compared to patients treated with warfarin. In these patients another anticoagulant should be used [see Dosage and Administration (2.1), Warnings and Precautions (5.1), and Clinical Studies (14.1)].
B. PREMATURE DISCONTINUATION OF SAVAYSA INCREASES THE RISK OF ISCHEMIC EVENTS
Premature discontinuation of any oral anticoagulant in the absence of adequate alternative anticoagulation increases the risk of ischemic events. If SAVAYSA is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant as described in the transition guidance [see Dosage and Administration (2.4), Warnings and Precautions (5.2), and Clinical Studies (14.1)].
CloseC. SPINAL/EPIDURAL HEMATOMA
Epidural or spinal hematomas may occur in patients treated with SAVAYSA who are receiving neuraxial anesthesia or undergoing spinal puncture. These hematomas may result in long-term or permanent paralysis. Consider these risks when scheduling patients for spinal procedures. Factors that can increase the risk of developing epidural or spinal hematomas in these patients include:
- use of indwelling epidural catheters
- concomitant use of other drugs that affect hemostasis, such as nonsteroidal anti-inflammatory drugs (NSAIDs), platelet inhibitors, other anticoagulants
- a history of traumatic or repeated epidural or spinal punctures
- a history of spinal deformity or spinal surgery
- optimal timing between the administration of SAVAYSA and neuraxial procedures is not known
[see Warnings and Precautions (5.4)].
Monitor patients frequently for signs and symptoms of neurological impairment. If neurological compromise is noted, urgent treatment is necessary [see Warnings and Precautions (5.4)].
Consider the benefits and risks before neuraxial intervention in patients anticoagulated or to be anticoagulated [see Warnings and Precautions (5.4)].
-
1 INDICATIONS AND USAGE1.1 Reduction in the Risk of Stroke and Systemic Embolism in Nonvalvular Atrial Fibrillation - SAVAYSA is indicated to reduce the risk of stroke and systemic embolism (SE) in patients with ...
1.1 Reduction in the Risk of Stroke and Systemic Embolism in Nonvalvular Atrial Fibrillation
SAVAYSA is indicated to reduce the risk of stroke and systemic embolism (SE) in patients with nonvalvular atrial fibrillation (NVAF).
Limitation of Use for NVAF
SAVAYSA should not be used in patients with CrCL > 95 mL/min because of an increased risk of ischemic stroke compared to warfarin [see Dosage and Administration (2.1), Warnings and Precautions (5.1) and Clinical Studies (14.1)].
Close1.2 Treatment of Deep Vein Thrombosis and Pulmonary Embolism
SAVAYSA is indicated for the treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE) following 5 to 10 days of initial therapy with a parenteral anticoagulant.
-
2 DOSAGE AND ADMINISTRATION2.1 Nonvalvular Atrial Fibrillation - The recommended dose of SAVAYSA is 60 mg taken orally once daily [see Warnings and Precautions (5.1) and Clinical Studies (14.1)]. Assess creatinine ...
2.1 Nonvalvular Atrial Fibrillation
The recommended dose of SAVAYSA is 60 mg taken orally once daily [see Warnings and Precautions (5.1) and Clinical Studies (14.1)]. Assess creatinine clearance, as calculated using the Cockcroft-Gault equation1, before initiating therapy with SAVAYSA. Do not use SAVAYSA in patients with CrCL > 95 mL/min.
Reduce SAVAYSA dose to 30 mg once daily in patients with CrCL 15 to 50 mL/min [see Use in Specific Populations (8.6), and Clinical Pharmacology (12.3)].
- 1
- Cockcroft-Gault CrCL = (140-age) × (weight in kg) × (0.85 if female) / (72 × creatinine in mg/dL).
2.2 Treatment of Deep Vein Thrombosis and Pulmonary Embolism
The recommended dose of SAVAYSA is 60 mg taken orally once daily following 5 to 10 days of initial therapy with a parenteral anticoagulant [see Clinical Studies (14.2)].
Reduce SAVAYSA dose to 30 mg once daily in patients with CrCL 15 to 50 mL/min, patients who weigh less than or equal to 60 kg, or patients who are taking certain concomitant P-gp inhibitor medications [see Clinical Studies (14.2)].
2.3 Administration Information
If a dose of SAVAYSA is missed, the dose should be taken as soon as possible on the same day. Dosing should resume the next day according to the normal dosing schedule. The dose should not be doubled to make up for a missed dose.
SAVAYSA can be taken without regard to food [see Clinical Pharmacology (12.3)].
2.4 Transition to or from SAVAYSA
Transition to SAVAYSA From To Recommendation Warfarin or other Vitamin K Antagonists SAVAYSA Discontinue warfarin and start SAVAYSA when the INR is ≤ 2.5 Oral anticoagulants other than warfarin or other Vitamin K Antagonists SAVAYSA Discontinue current oral anticoagulant and start SAVAYSA at the time of the next scheduled dose of the other oral anticoagulant Low Molecular Weight Heparin (LMWH) SAVAYSA Discontinue LMWH and start SAVAYSA at the time of the next scheduled administration of LMWH Unfractionated heparin SAVAYSA Discontinue the infusion and start SAVAYSA 4 hours later Transition from SAVAYSA From To Recommendation Abbreviations: INR=International Normalized Ratio SAVAYSA Warfarin Oral option: For patients taking 60 mg of SAVAYSA, reduce the dose to 30 mg and begin warfarin concomitantly. For patients receiving 30 mg of SAVAYSA, reduce the dose to 15 mg and begin warfarin concomitantly. INR must be measured at least weekly and just prior to the daily dose of SAVAYSA to minimize the influence of SAVAYSA on INR measurements. Once a stable INR ≥ 2.0 is achieved, SAVAYSA should be discontinued and the warfarin continued SAVAYSA Warfarin Parenteral option: Discontinue SAVAYSA and administer a parenteral anticoagulant and warfarin at the time of the next scheduled SAVAYSA dose. Once a stable INR ≥ 2.0 is achieved the parenteral anticoagulant should be discontinued and the warfarin continued SAVAYSA Non-Vitamin-K-Dependent Oral anticoagulants Discontinue SAVAYSA and start the other oral anticoagulant at the time of the next dose of SAVAYSA SAVAYSA Parenteral anticoagulants Discontinue SAVAYSA and start the parenteral anticoagulant at the time of the next dose of SAVAYSA 2.5 Discontinuation for Surgery and Other Interventions
Discontinue SAVAYSA at least 24 hours before invasive or surgical procedures because of the risk of bleeding [see Warnings and Precautions (5.3)].
If surgery cannot be delayed, there is an increased risk of bleeding. This risk of bleeding should be weighed against the urgency of intervention [see Warnings and Precautions (5.3)].
SAVAYSA can be restarted after the surgical or other procedure as soon as adequate hemostasis has been established noting that the time to onset of pharmacodynamic effect is 1-2 hours [see Warnings and Precautions (5.2)]. Administer a parenteral anticoagulant and then switch to oral SAVAYSA, if oral medication cannot be taken during or after surgical intervention.
Close2.6 Administration Options
For patients who are unable to swallow whole tablets, SAVAYSA tablets may be crushed and mixed with 2 to 3 ounces of water and immediately administered by mouth or through a gastric tube. The crushed tablets may also be mixed into applesauce and immediately administered orally [see Clinical Pharmacology (12.3)].
-
3 DOSAGE FORMS AND STRENGTHS60 mg, yellow round shaped, film-coated tablets, debossed with DSC L60 on one side - 30 mg, pink round shaped, film-coated tablets, debossed with DSC L30 on one side - 15 mg, orange round shaped ...
- 60 mg, yellow round shaped, film-coated tablets, debossed with DSC L60 on one side
- 30 mg, pink round shaped, film-coated tablets, debossed with DSC L30 on one side
- 15 mg, orange round shaped, film-coated tablets, debossed with DSC L15 on one side
-
4 CONTRAINDICATIONSSAVAYSA is contraindicated in patients with: Active pathological bleeding [see Warnings and Precautions (5.3) and Adverse Reactions (6.1)].
SAVAYSA is contraindicated in patients with:
- Active pathological bleeding [see Warnings and Precautions (5.3) and Adverse Reactions (6.1)].
-
5 WARNINGS AND PRECAUTIONS5.1 Reduced Efficacy in Nonvalvular Atrial Fibrillation Patients with CrCL > 95 mL/min - SAVAYSA should not be used in patients with CrCL > 95 mL/min. In the randomized ENGAGE AF-TIMI 48 study ...
5.1 Reduced Efficacy in Nonvalvular Atrial Fibrillation Patients with CrCL > 95 mL/min
SAVAYSA should not be used in patients with CrCL > 95 mL/min. In the randomized ENGAGE AF-TIMI 48 study, NVAF patients with CrCL > 95 mL/min had an increased rate of ischemic stroke with SAVAYSA 60 mg daily compared to patients treated with warfarin. In these patients another anticoagulant should be used [see Dosage and Administration (2.1) and Clinical Studies (14.1)].
5.2 Increased Risk of Stroke with Discontinuation of SAVAYSA in Patients with Nonvalvular Atrial Fibrillation
Premature discontinuation of any oral anticoagulant in the absence of adequate alternative anticoagulation increases the risk of ischemic events. If SAVAYSA is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant as described in the transition guidance [see Dosage and Administration (2.4) and Clinical Studies (14.1)].
5.3 Risk of Bleeding
SAVAYSA increases the risk of bleeding and can cause serious and potentially fatal bleeding. Promptly evaluate any signs or symptoms of blood loss.
Discontinue SAVAYSA in patients with active pathological bleeding.
Concomitant use of drugs affecting hemostasis may increase the risk of bleeding. These include aspirin and other antiplatelet agents, other antithrombotic agents, fibrinolytic therapy, chronic use of nonsteroidal anti-inflammatory drugs (NSAIDs), selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) [see Drug Interactions (7.1)].
Reversal of Anticoagulant Effect
There is no established way to reverse the anticoagulant effects of SAVAYSA, which can be expected to persist for approximately 24 hours after the last dose. The anticoagulant effect of SAVAYSA cannot be reliably monitored with standard laboratory testing. A specific reversal agent for edoxaban is not available. Hemodialysis does not significantly contribute to edoxaban clearance [see Clinical Pharmacology (12.3)]. Protamine sulfate, vitamin K, and tranexamic acid are not expected to reverse the anticoagulant activity of SAVAYSA. The use of prothrombin complex concentrates (PCC), or other procoagulant reversal agents such as activated prothrombin complex concentrate (APCC) or recombinant factor VIIa (rFVIIa) may be considered but has not been evaluated in clinical outcome studies [see Clinical Pharmacology (12.2)]. When PCCs are used, monitoring for anticoagulation effect of edoxaban using clotting test (PT, INR, or aPTT) or anti-FXa activity is not useful and is not recommended.
5.4 Spinal/Epidural Anesthesia or Puncture
When neuraxial anesthesia (spinal/epidural anesthesia) or spinal/epidural puncture is employed, patients treated with antithrombotic agents for prevention of thromboembolic complications are at risk of developing an epidural or spinal hematoma, which can result in long-term or permanent paralysis.
The risk of these events may be increased by the postoperative use of indwelling epidural catheters or the concomitant use of medicinal products affecting hemostasis. Indwelling epidural or intrathecal catheters should not be removed earlier than 12 hours after the last administration of SAVAYSA. The next dose of SAVAYSA should not be administered earlier than 2 hours after the removal of the catheter. The risk may also be increased by traumatic or repeated epidural or spinal puncture.
Monitor patients frequently for signs and symptoms of neurological impairment (e.g., numbness or weakness of the legs, bowel, or bladder dysfunction). If neurological compromise is noted, urgent diagnosis and treatment is necessary. Prior to neuraxial intervention the physician should consider the potential benefit versus the risk in anticoagulated patients or in patients to be anticoagulated for thromboprophylaxis.
5.5 Patients with Mechanical Heart Valves or Moderate to Severe Mitral Stenosis
The safety and efficacy of SAVAYSA has not been studied in patients with mechanical heart valves or moderate to severe mitral stenosis. The use of SAVAYSA is not recommended in these patients [see Clinical Studies (14.1)].
Close5.6 Increased Risk of Thrombosis in Patients with Triple Positive Antiphospholipid Syndrome
Direct-acting oral anticoagulants (DOACs), including SAVAYSA, are not recommended for use in patients with triple positive antiphospholipid syndrome (APS). For patients with APS (especially those who are triple positive [positive for lupus anticoagulant, anticardiolipin antibodies, and anti-beta 2-glycoprotein I antibodies]), treatment with DOACs has been associated with increased rates of recurrent thrombotic events compared with vitamin K antagonist therapy.
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are discussed in greater detail in other sections of the prescribing information. Increased Risk of Stroke with Discontinuation of SAVAYSA ...
The following clinically significant adverse reactions are discussed in greater detail in other sections of the prescribing information.
- Increased Risk of Stroke with Discontinuation of SAVAYSA in Patients with Nonvalvular Atrial Fibrillation [see Warnings and Precautions (5.2)]
- Risk of Bleeding [see Warnings and Precautions (5.3)]
- Spinal/Epidural Anesthesia or Puncture [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of SAVAYSA was evaluated in the ENGAGE AF-TIMI 48, Hokusai VTE, and Hokusai VTE Cancer studies including 11,530 patients exposed to SAVAYSA 60 mg and 7124 patients exposed to SAVAYSA 30 mg once daily [see Clinical Studies (14)].
The ENGAGE AF-TIMI 48 Study
In the ENGAGE AF-TIMI 48 study, the median study drug exposure for the SAVAYSA and warfarin treatment groups was 2.5 years.
Bleeding was the most common reason for treatment discontinuation. Bleeding led to treatment discontinuation in 3.9% and 4.1% of patients in the SAVAYSA 60 mg and warfarin treatment groups, respectively.
In the overall population, major bleeding was lower in the SAVAYSA group compared to the warfarin group [HR 0.80 (0.70, 0.91), p < 0.001]. Table 6.1 shows major bleeding events (percentage of patients with at least one bleeding event, per year) for the indicated population (CrCL ≤ 95 mL/min).
Table 6.1: Adjudicated Bleeding Events for NVAF Patients with CrCL ≤ 95 mL/min* Event† SAVAYSA 60 mg‡
N = 5417
n (%/year)Warfarin
N = 5485
n (%/year)SAVAYSA 60 mg vs. Warfarin
HR (95% CI)Abbreviations: HR = Hazard Ratio versus Warfarin, CI = Confidence Interval, n = number of patients with events, N = number of patients in Safety population, - *
- The on-treatment period is during treatment or within 2 days of stopping study treatment. The difference in hemorrhagic stroke rate from Table 14.1 is because Table 14.1 includes events occurring during treatment or within 3 days of stopping study treatment and this table only includes patients with CrCL ≤ 95 mL/min.
- †
- A subject can be included in multiple sub-categories if he/she had an event for those categories.
- ‡
- Includes all patients with CrCL ≤ 95 mL/min randomized to receive 60 mg once daily, including those who were dose-reduced to 30 mg once daily because of prespecified baseline conditions.
- §
- A major bleeding event (the study primary safety endpoint) was defined as clinically overt bleeding that met one of the following criteria: fatal bleeding; symptomatic bleeding in a critical site such as retroperitoneal, intracranial, intraocular, intraspinal, intra-articular, pericardial, or intramuscular with compartment syndrome; a clinically overt bleeding event that caused a fall in hemoglobin of at least 2.0 g/dL (or a fall in hematocrit of at least 6.0% in the absence of hemoglobin data), when adjusted for transfusions (1 unit of transfusion = 1.0 g/dL drop in hemoglobin).
- ¶
- ICH includes primary hemorrhagic stroke, subarachnoid hemorrhage, epidural/subdural hemorrhage, and ischemic stroke with major hemorrhagic conversion.
- #
- Gastrointestinal (GI) bleeds include bleeding from upper and lower GI tract. Lower GI tract bleeding includes rectal bleeds.
- Þ
- Fatal bleed is a bleeding event during the on-treatment period and adjudicated as leading directly to death within 7 days.
Major Bleeding§ 357 (3.1) 431 (3.7) 0.84 (0.73, 0.97) Intracranial Hemorrhage (ICH)¶ 53 (0.5) 122 (1.0) 0.44 (0.32, 0.61) Hemorrhagic Stroke 33 (0.3) 69 (0.6) 0.49 (0.32, 0.74) Other ICH 20 (0.2) 55 (0.5) 0.37 (0.22, 0.62) Gastrointestinal# 205 (1.8) 150 (1.3) 1.40 (1.13, 1.73) Fatal BleedingÞ 21 (0.2) 42 (0.4) 0.51 (0.30, 0.86) ICH 19 (0.2) 36 (0.3) 0.54 (0.31, 0.94) Non-intracranial 2 (< 0.1) 6 (< 0.1) ---- The most common site of a major bleeding event was the gastrointestinal (GI) tract. Table 6.2 shows the number of and the rate at which patients experienced GI bleeding in the SAVAYSA 60 mg and warfarin treatment groups.
Table 6.2: Gastrointestinal Bleeding Events for NVAF Patients with CrCL ≤ 95 mL/min* SAVAYSA
N = 5417
n (%/year)Warfarin
N = 5485
n (%/year)Major Gastrointestinal (GI) Bleeding† 205 (1.78) 150 (1.27) Upper GI 123 (1.06) 88 (0.74) Lower GI‡ 85 (0.73) 64 (0.54) GUSTO§ Severe GI bleeding 16 (0.14) 17 (0.14) Fatal GI bleeding 1 (< 0.1) 2 (< 0.1) The rate of anemia-related adverse events was greater with SAVAYSA 60 mg than with warfarin (9.6% vs. 6.8%).
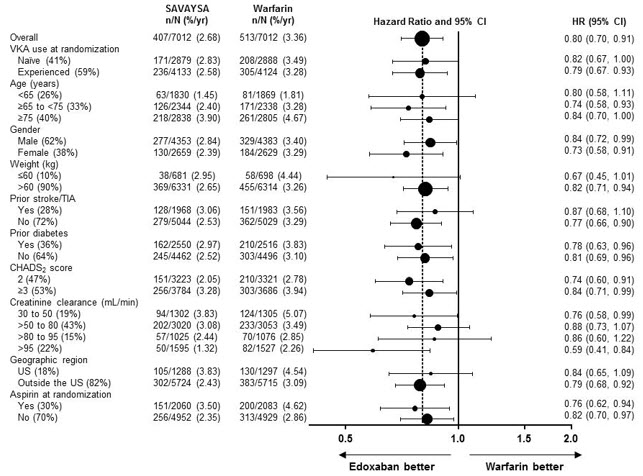
The comparative rates of major bleeding on SAVAYSA and warfarin were generally consistent among subgroups (see Figure 6.1). Bleeding rates appeared higher in both treatment arms (SAVAYSA and warfarin) in the following subgroups of patients: those receiving aspirin, those in the United States, those more than 75 years old and those with reduced renal function.
Figure 6.1: Adjudicated Major Bleeding in the ENGAGE AF-TIMI 48* Study Note: The figure above presents effects in various subgroups all of which are baseline characteristics and most of which were pre-specified. The 95% confidence limits that are shown do not take into account how many comparisons were made, nor do they reflect the effect of a particular factor after adjustment for all other factors. Apparent homogeneity or heterogeneity among groups should not be over-interpreted. - *
- During or within 2 days of stopping study treatment
Other Adverse Reactions
The most common non-bleeding adverse reactions (≥ 1%) for SAVAYSA 60 mg versus warfarin were rash (4.2% vs. 4.1%), and abnormal liver function tests (4.8% vs. 4.6%), respectively.
Interstitial Lung Disease (ILD) was reported as a serious adverse event on treatment for SAVAYSA 60 mg and warfarin in 15 (0.2%) and 7 (0.1%) patients, respectively. Many of the cases in both treatment groups were confounded by the use of amiodarone, which has been associated with ILD, or by infectious pneumonia. In the overall study period, there were 5 and 0 fatal ILD cases in the SAVAYSA 60 mg and warfarin groups, respectively.
The Hokusai VTE Study
The safety of SAVAYSA in the treatment of VTE was assessed in the Hokusai VTE study. The duration of drug exposure for SAVAYSA was ≤ 6 months for 1561 (37.9%) of patients, > 6 months for 2557 (62.1%) of patients and 12 months for 1661 (40.3%) of patients.
Bleeding was the most common reason for treatment discontinuation and occurred in 1.4% and 1.4% of patients in the SAVAYSA and warfarin arms, respectively.
Bleeding in Patients with DVT and/or PE in the Hokusai VTE Study
The major safety outcome was Clinically Relevant Bleeding, defined as the composite of Major and Clinically Relevant Non-Major (CRNM) Bleeding that occurred during or within three days of stopping study treatment. The incidence of Clinically Relevant Bleeding was lower in SAVAYSA than warfarin [HR (95% CI): 0.81 (0.71, 0.94); p = 0.004].
Table 6.3 shows the number of patients experiencing bleeding events in the Hokusai VTE Study.
Table 6.3: Bleeding Events in the Hokusai VTE Study SAVAYSA
(N = 4118)Warfarin
(N = 4122)Abbreviations: N = number of patients in the modified intent-to-treat population; n = number of events; CRNM = clinically relevant non-major - *
- Primary Safety Endpoint: Clinically Relevant Bleeding (composite of Major and CRNM).
- †
- A major bleeding event was defined as clinically overt bleeding that met one of the following criteria: associated with a fall in hemoglobin level of 2.0 g/dL or more, or leading to transfusion of two or more units of packed red cells or whole blood; occurring in a critical site or organ: intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome, retroperitoneal; contributing to death.
- ‡
- CRNM bleeding was defined as overt bleeding not meeting the criteria for a major bleeding event but that was associated with a medical intervention, an unscheduled contact (visit or telephone call) with a physician, temporary cessation of study treatment, or associated with discomfort for the subject such as pain, or impairment of activities of daily life.
Clinically Relevant Bleeding* (Major/CRNM), n (%) 349 (8.5) 423 (10.3) Major Bleeding†, n (%) 56 (1.4) 66 (1.6) Fatal bleeding 2 (<0.1) 10 (0.2) Intracranial fatal 0 (0.0) 6 (0.1) Non-fatal critical organ bleeding 13 (0.3) 25 (0.6) Intracranial bleeding 5 (0.1) 12 (0.3) Non-fatal non-critical organ bleeding 41 (1.0) 33 (0.8) Decrease in Hb ≥ 2 g/dL 40 (1.0) 33 (0.8) Transfusion of ≥ 2 units of RBC 28 (0.7) 22 (0.5) CRNM Bleeding‡ 298 (7.2) 368 (8.9) Any Bleed 895 (21.7) 1056 (25.6) Patients with low body weight (≤ 60 kg), CrCL ≤ 50 mL/min, or concomitant use of select P-gp inhibitors were randomized to receive SAVAYSA 30 mg or warfarin. As compared to all patients who received SAVAYSA or warfarin in the 60 mg cohort, all patients who received SAVAYSA or warfarin in the 30 mg cohort (n = 1452, 17.6% of the entire study population) were older (60.1 vs 54.9 years), more frequently female (66.5% vs 37.7%), more frequently of Asian race (46.0% vs 15.6%) and had more co-morbidities (e.g., history of bleeding, hypertension, diabetes, cardiovascular disease, cancer). Clinically relevant bleeding events occurred in 58/733 (7.9%) of the SAVAYSA patients receiving 30 mg once daily and 92/719 (12.8%) of warfarin patients meeting the above criteria.
In the Hokusai VTE study, among all patients the most common bleeding adverse reactions (≥ 1%) are shown in Table 6.4.
Table 6.4: Adverse Reactions Occurring in ≥ 1% of Patients Treated in Hokusai VTE SAVAYSA 60 mg
(N = 4118)
n (%)Warfarin
(N = 4122)
n (%)Bleeding ADRs* Vaginal† 158 (9) 126 (7.1) Cutaneous soft tissue 245 (5.9) 414 (10) Epistaxis 195 (4.7) 237 (5.7) Gastrointestinal bleeding 171 (4.2) 150 (3.6) Lower gastrointestinal 141 (3.4) 126 (3.1) Oral/pharyngeal 138 (3.4) 162 (3.9) Macroscopic hematuria/urethral 91 (2.2) 117 (2.8) Puncture site 56 (1.4) 99 (2.4) Non-Bleeding ADRs Rash 147 (3.6) 151 (3.7) Abnormal liver function tests 322 (7.8) 322 (7.8) Anemia 72 (1.7) 55 (1.3) Bleeding in Patients with VTE in the Hokusai VTE Cancer Study
The safety of SAVAYSA in patients with cancer and VTE was evaluated in the Hokusai VTE Cancer study [see Clinical Studies (14.2)]. The median duration of SAVAYSA exposure was 211 days (range, 2 to 423). The safety outcome was major bleeding that occurred during or within three days of stopping study treatment. The incidence of major bleeding was higher in the SAVAYSA arm than in the dalteparin arm [HR (95% CI): 2.00 (1.09, 3.66)].
Table 6.5 presents the bleeding results from the Hokusai VTE Cancer study.
Table 6.5: Bleeding Events in the Hokusai VTE Cancer Study SAVAYSA
(N = 522)Dalteparin
(N = 524)Abbreviations: N = number of patients in the modified intent-to-treat population; n = number of events; CRNM = clinically relevant non-major - *
- A major bleeding event was defined as clinically overt bleeding that met one of the following criteria: associated with a fall in hemoglobin level of 2.0 g/dL or more, or leading to transfusion of two or more units of packed red cells or whole blood; occurring in a critical site or organ: intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome, retroperitoneal; contributing to death.
- †
- All events in this table, except for the fatal bleeding event on SAVAYSA, are based on adjudicated events. The fatal bleeding event on SAVAYSA was adjudicated as a major bleed; however, the adjudicated cause of death was cancer-related death.
- ‡
- CRNM bleeding was defined as overt bleeding not meeting the criteria for a major bleeding event but that was associated with a medical intervention, an unscheduled contact (visit or telephone call) with a physician, temporary cessation of study treatment, or associated with discomfort for the subject such as pain or impairment of activities of daily life.
Major Bleeding*, n (%) 32 (6.1%) 16 (3.1%) Fatal bleeding 1 (0.2%)† 2 (0.4%) Intracranial 0 1 (0.2%) Lower gastrointestinal 1 (0.2%) 1 (0.2%) Non-fatal critical organ bleeding 5 (1%) 6 (1.1%) Intracranial bleeding 2 (0.4%) 2 (0.4%) Non-fatal non-critical organ bleeding 27 (5.2%) 8 (1.5%) Gastrointestinal 22 (4.2%) 4 (0.8%) Upper gastrointestinal 18 (3.4%) 3 (0.6%) Lower gastrointestinal 3 (0.6%) 1 (0.2%) Decrease in Hb ≥ 2 g/dL 28 (5.4%) 11 (2.1%) CRNM Bleeding‡, n (%) 70 (13.4%) 48 (9.2%) Any Bleeding, n (%) 137 (26.2%) 104 (19.8%) In patients with GI cancer at randomization, major bleeding occurred in 13.2% (18/136) in the SAVAYSA group and 2.4% (3/125) in the dalteparin group. In patients without GI cancer at randomization, major bleeding occurred in 3.6% (14/386) in the SAVAYSA group and 3.3% (13/399) in the dalteparin group.
Close6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of SAVAYSA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders: thrombocytopenia
Gastrointestinal disorders: abdominal pain
Immune system disorders: angioedema, hypersensitivity
Nervous system disorders: dizziness, headache
Renal and urinary disorders: anticoagulant-related nephropathy
Skin and subcutaneous tissue disorders: urticaria
-
7 DRUG INTERACTIONS7.1 Anticoagulants, Antiplatelets, Thrombolytics, and SSRIs/SNRIs - Co-administration of anticoagulants, antiplatelet drugs, thrombolytics and SSRIs or SNRIs may increase the risk of bleeding ...
7.1 Anticoagulants, Antiplatelets, Thrombolytics, and SSRIs/SNRIs
Co-administration of anticoagulants, antiplatelet drugs, thrombolytics and SSRIs or SNRIs may increase the risk of bleeding. Promptly evaluate any signs or symptoms of blood loss if patients are treated concomitantly with anticoagulants, aspirin, other platelet aggregation inhibitors, and/or NSAIDs [see Warnings and Precautions (5.3)].
Long-term concomitant treatment with SAVAYSA and other anticoagulants is not recommended because of increased risk of bleeding [see Warnings and Precautions (5.3)]. Short term co-administration may be needed for patients transitioning to or from SAVAYSA [see Dosage and Administration (2.4)].
In clinical studies with SAVAYSA concomitant use of aspirin (low dose ≤ 100 mg/day) or thienopyridines, and NSAIDs was permitted and resulted in increased rates of Clinically Relevant Bleeding. Carefully monitor for bleeding in patients who require chronic treatment with low dose aspirin and/or NSAIDs [see Warnings and Precautions (5.3) and Clinical Pharmacology (12.3)].
As with other anticoagulants the possibility may exist that patients are at an increased risk of bleeding in case of concomitant use with SSRIs or SNRIs due to their reported effect on platelets [see Warnings and Precautions (5.3)].
7.2 P-gp Inducers
Avoid the concomitant use of SAVAYSA with rifampin [see Clinical Pharmacology (12.3)].
Close7.3 P-gp Inhibitors
Treatment of NVAF
Based on clinical experience from the ENGAGE AF-TIMI 48 study, dose reduction in patients concomitantly receiving P-gp inhibitors resulted in edoxaban blood levels that were lower than in patients who were given the full dose. Consequently, no dose reduction is recommended for concomitant P-gp inhibitor use [see Dosage and Administration (2.1), Clinical Pharmacology (12.3), and Clinical Studies (14.1)].
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Available data about SAVAYSA use in pregnant women are insufficient to determine whether there are drug-associated risks for adverse developmental outcomes. In ...
8.1 Pregnancy
Risk Summary
Available data about SAVAYSA use in pregnant women are insufficient to determine whether there are drug-associated risks for adverse developmental outcomes. In animal developmental studies, no adverse developmental effects were seen when edoxaban was administered orally to pregnant rats and rabbits during organogenesis at up to 16-times and 8-times, respectively, the human exposure, when based on body surface area and AUC, respectively (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Pregnancy confers an increased risk of thromboembolism that is higher for women with underlying thromboembolic disease and certain high-risk pregnancy conditions. Published data describe that women with a previous history of venous thrombosis are at high risk for recurrence during pregnancy.
Fetal/Neonatal adverse reactions
Use of anticoagulants, including edoxaban, may increase the risk of bleeding in the fetus and neonate. Monitor neonates for bleeding [see Warnings and Precautions (5.3)].
Labor or delivery
All patients receiving anticoagulants, including pregnant women, are at risk for bleeding. SAVAYSA use during labor or delivery in women who are receiving neuraxial anesthesia may result in epidural or spinal hematomas. Consider use of a shorter acting anticoagulant as delivery approaches [see Warnings and Precautions (5.3)].
Data
Animal Data
Embryo-fetal development studies were conducted in pregnant rats and rabbits during the period of organogenesis. In rats, no malformation was seen when edoxaban was administered orally at doses up to 300 mg/kg/day, or 49 times the human dose of 60 mg/day normalized to body surface area. Increased post-implantation loss occurred at 300 mg/kg/day, but this effect may be secondary to the maternal vaginal hemorrhage seen at this dose. In rabbits, no malformation was seen at doses up to 600 mg/kg/day (49 times the human exposure at a dose of 60 mg/day when based on AUC). Embryo-fetal toxicities occurred at maternally toxic doses, and included absent or small fetal gallbladder at 600 mg/kg/day, and increased post-implantation loss, increased spontaneous abortion, and decreased live fetuses and fetal weight at doses equal to or greater than 200 mg/kg/day, which is equal to or greater than 20 times the human exposure.
In a rat pre- and post-natal developmental study, edoxaban was administered orally during the period of organogenesis and through lactation day 20 at doses up to 30 mg/kg/day, which is up to 3 times the human exposure when based on AUC. Vaginal bleeding in pregnant rats and delayed avoidance response (a learning test) in female offspring were seen at 30 mg/kg/day.
8.2 Lactation
Risk Summary
There are no data on the presence of edoxaban in human milk, or its effects on the breastfeeding infant or on milk production. Edoxaban was present in rat milk. Because of the potential for serious adverse reactions in nursing infants, including hemorrhage, advise patients that breastfeeding is not recommended during treatment with SAVAYSA.
8.3 Females and Males of Reproductive Potential
Females of reproductive potential requiring anticoagulation should discuss pregnancy planning with their physician.
The risk of clinically significant uterine bleeding, potentially requiring gynecological surgical interventions, identified with oral anticoagulants including SAVAYSA should be assessed in females of reproductive potential and those with abnormal uterine bleeding.
8.4 Pediatric Use
The safety and effectiveness of SAVAYSA have not been established in pediatric patients with confirmed VTE (PE and/or DVT). Effectiveness was not demonstrated in an adequate and well-controlled study conducted in 145 SAVAYSA-treated pediatric patients, from birth to less than 18 years of age with confirmed VTE (PE and/or DVT), treated for 3 months up to a maximum of 12 months.
8.5 Geriatric Use
Of the total patients in the ENGAGE AF-TIMI 48 study, 5182 (74%) were 65 years and older, while 2838 (41%) were 75 years and older. In Hokusai VTE, 1334 (32%) patients were 65 years and older, while 560 (14%) patients were 75 years and older. In the Hokusai VTE Cancer Study, 539 (52%) patients were 65 years and older and 176 (17%) were 75 years and older. In clinical trials the efficacy and safety of SAVAYSA in elderly (65 years or older) and younger patients were similar [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14)].
8.6 Renal Impairment
Renal clearance accounts for approximately 50% of the total clearance of edoxaban. Consequently, edoxaban blood levels are increased in patients with poor renal function compared to those with higher renal function. Reduce SAVAYSA dose to 30 mg once daily in patients with CrCL 15-50 mL/min. There are limited clinical data with SAVAYSA in patients with CrCL < 15 mL/min; SAVAYSA is therefore not recommended in these patients. Hemodialysis does not significantly contribute to SAVAYSA clearance [see Dosage and Administration (2.1, 2.2) and Clinical Pharmacology (12.3)].
As renal function improves and edoxaban blood levels decrease, the risk for ischemic stroke increases in patients with NVAF [see Indications and Usage (1.1), Dosage and Administration (2.1), and Clinical Studies (14.1)].
8.7 Hepatic Impairment
The use of SAVAYSA in patients with moderate or severe hepatic impairment (Child-Pugh B and C) is not recommended as these patients may have intrinsic coagulation abnormalities. No dose reduction is required in patients with mild hepatic impairment (Child-Pugh A) [see Clinical Pharmacology (12.3)].
Close8.8 Low Body Weight Consideration for Patients Treated for DVT and/or PE
Based on the clinical experience from the Hokusai VTE study, reduce SAVAYSA dose to 30 mg in patients with body weight less than or equal to 60 kg [see Dosage and Administration (2.2) and Clinical Studies (14.2)].
-
10 OVERDOSAGEA specific reversal agent for edoxaban is not available. Overdose of SAVAYSA increases the risk of bleeding. The following are not expected to reverse the anticoagulant effects of edoxaban ...
A specific reversal agent for edoxaban is not available. Overdose of SAVAYSA increases the risk of bleeding.
The following are not expected to reverse the anticoagulant effects of edoxaban: protamine sulfate, vitamin K, and tranexamic acid [see Warnings and Precautions (5.3)].
Hemodialysis does not significantly contribute to edoxaban clearance [see Clinical Pharmacology (12.3)].
Close -
11 DESCRIPTIONEdoxaban, a factor Xa inhibitor, is supplied as edoxaban tosylate monohydrate. The chemical name is ...
Edoxaban, a factor Xa inhibitor, is supplied as edoxaban tosylate monohydrate. The chemical name is N-(5-Chloropyridin-2-yl)-N'-[(1S,2R,4S)-4-(N,N-dimethylcarbamoyl)-2-(5-methyl-4,5,6,7-tetrahydro[1,3]thiazolo[5,4-c]pyridine-2-carboxamido)cyclohexyl] oxamide mono (4-methylbenzenesulfonate) monohydrate. Edoxaban tosylate monohydrate has the empirical formula C24H30ClN7O4S∙C7H8O3S∙H2O representing a molecular weight of 738.27. The chemical structure of edoxaban tosylate monohydrate is:
It is a white to pale yellowish-white crystalline powder. The solubility of edoxaban tosylate (pKa 6.7) decreases with increasing pH. It is slightly soluble in water, pH 3 to 5 buffer, very slightly soluble at pH 6 to 7; and practically insoluble at pH 8 to 9.
SAVAYSA is available for oral administration as a 60 mg, 30 mg, or 15 mg round shaped, film-coated tablet, debossed with product identification markings. Each 60 mg tablet contains 80.82 mg edoxaban tosylate monohydrate equivalent to 60 mg of edoxaban. Each 30 mg tablet contains 40.41 mg edoxaban tosylate monohydrate equivalent to 30 mg of edoxaban. Each 15 mg tablet contains 20.20 mg edoxaban tosylate monohydrate equivalent to 15 mg of edoxaban. The inactive ingredients are: mannitol, pregelatinized starch, crospovidone, hydroxypropyl cellulose, magnesium stearate, talc, and carnauba wax. The color coatings contain hypromellose, titanium dioxide, talc, polyethylene glycol 8000, iron oxide yellow (60 mg tablets and 15 mg tablets), and iron oxide red (30 mg tablets and 15 mg tablets).
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Edoxaban is a selective inhibitor of FXa. It does not require antithrombin III for antithrombotic activity. Edoxaban inhibits free FXa, and prothrombinase activity and ...
12.1 Mechanism of Action
Edoxaban is a selective inhibitor of FXa. It does not require antithrombin III for antithrombotic activity. Edoxaban inhibits free FXa, and prothrombinase activity and inhibits thrombin-induced platelet aggregation. Inhibition of FXa in the coagulation cascade reduces thrombin generation and reduces thrombus formation.
12.2 Pharmacodynamics
As a result of FXa inhibition, edoxaban prolongs clotting time tests such as prothrombin time (PT), and activated partial thromboplastin time (aPTT). Changes observed in PT, INR, and aPTT at the expected therapeutic dose, however, are small, subject to a high degree of variability and not useful in monitoring the anticoagulant effect of edoxaban. Following oral administration, peak pharmacodynamic effects are observed within 1-2 hours, which correspond with peak edoxaban concentrations (Cmax).
Cardiac Electrophysiology
In a thorough QT study in healthy men and women aged 19-45 years, no QTc interval prolongation was observed with edoxaban (90 mg and 180 mg).
Effect of PCCs on Pharmacodynamics of SAVAYSA
There is no systematic evaluation of bleeding reversal by 4-factor prothrombin complex concentrate (PCC) products in patients who have received SAVAYSA.
Effects of PCC (50 IU/kg) on the pharmacodynamics of edoxaban were studied in healthy subjects following a punch biopsy. Following administration of a single dose of edoxaban, endogenous thrombin potential (ETP) returned to pre-edoxaban baseline levels in 0.5 hours after the initiation of a 15-minute infusion of 50 IU/kg PCC, compared to more than 24 hours with placebo. Mean ETP levels continued to increase and exceeded pre-edoxaban baseline, reaching maximum elevations (~40% over pre-edoxaban levels) at 22 hours after initiating PCC dose, which was the last observation of ETP. The clinical relevance of this ETP increase is unknown.
Close12.3 Pharmacokinetics
Edoxaban displays approximately dose-proportional pharmacokinetics for doses of 15 to 150 mg and 60 to 120 mg following single and repeat doses, respectively, in healthy subjects.
Absorption
Following oral administration, peak plasma edoxaban concentrations are observed within 1-2 hours. Absolute bioavailability is 62%. Food does not affect total systemic exposure to edoxaban. SAVAYSA was administered with or without food in the ENGAGE AF-TIMI 48 and Hokusai VTE trials.
Administration of a crushed 60 mg tablet, either mixed into applesauce or suspended in water and given through a nasogastric tube, showed similar exposure compared to administration of an intact tablet.
Distribution
Disposition is biphasic. The steady-state volume of distribution (Vdss) is 107 (19.9) L [mean (SD)]. In vitro plasma protein binding is approximately 55%. There is no clinically relevant accumulation of edoxaban (accumulation ratio 1.14) with once daily dosing.
Steady-state concentrations are achieved within 3 days.
Metabolism
Unchanged edoxaban is the predominant form in plasma. There is minimal metabolism via hydrolysis (mediated by carboxylesterase 1), conjugation, and oxidation by CYP3A4.
The predominant metabolite M-4, formed by hydrolysis, is human-specific and active and reaches less than 10% of the exposure of the parent compound in healthy subjects. Exposure to the other metabolites is less than 5% of exposure to edoxaban.
Elimination
Edoxaban is eliminated primarily as unchanged drug in the urine. Renal clearance (11 L/hour) accounts for approximately 50% of the total clearance of edoxaban (22 L/hour). Metabolism and biliary/intestinal excretion account for the remaining clearance. The terminal elimination half-life of edoxaban following oral administration is 10 to 14 hours.
Specific Populations
Hepatic Impairment
In a dedicated pharmacokinetic study, patients with mild or moderate hepatic impairment (classified as Child-Pugh A or Child-Pugh B) exhibited similar pharmacokinetics and pharmacodynamics to their matched healthy control group. There is no clinical experience with edoxaban in patients with severe hepatic impairment [see Use in Specific Populations (8.7)].
Renal Impairment
In a dedicated pharmacokinetic study, total systemic exposure to edoxaban for subjects with CrCL > 50 to < 80 mL/min, CrCL 30 to 50 mL/min, CrCL < 30 mL/min, or undergoing peritoneal dialysis, were increased by 32%, 74%, 72%, and 93%, respectively, relative to subjects with CrCL ≥ 80 mL/min [see Use in Specific Populations (8.6)].
Age
In a population pharmacokinetic analysis, after taking renal function and body weight into account, age had no additional clinically significant effect on edoxaban pharmacokinetics.
Weight
In a population pharmacokinetic analysis, total exposure in patients with median low body weight (55 kg) was increased by 13% as compared with patients with median high body weight (84 kg).
Drug Interactions
In vitro Drug Interactions Studies
In vitro studies indicate that edoxaban does not inhibit the major cytochrome P450 enzymes (CYP1A2, 2A6, 2B6, 2C8/9, 2C19, 2D6, 2E1, or 3A4) and does not induce CYP1A2, CYP3A4 or the P-gp transporter (MDR1). In vitro data also indicate that edoxaban does not inhibit the following transporters at clinically relevant concentrations: P-gp, the organic anion transporters OAT1 or OAT3; the organic cation transporters OCT1 or OCT2; or the organic ion transporting polypeptides OATP1B1 or OATP1B3. Edoxaban is a substrate of P-gp transporter.
Impact of Other Drugs on SAVAYSA
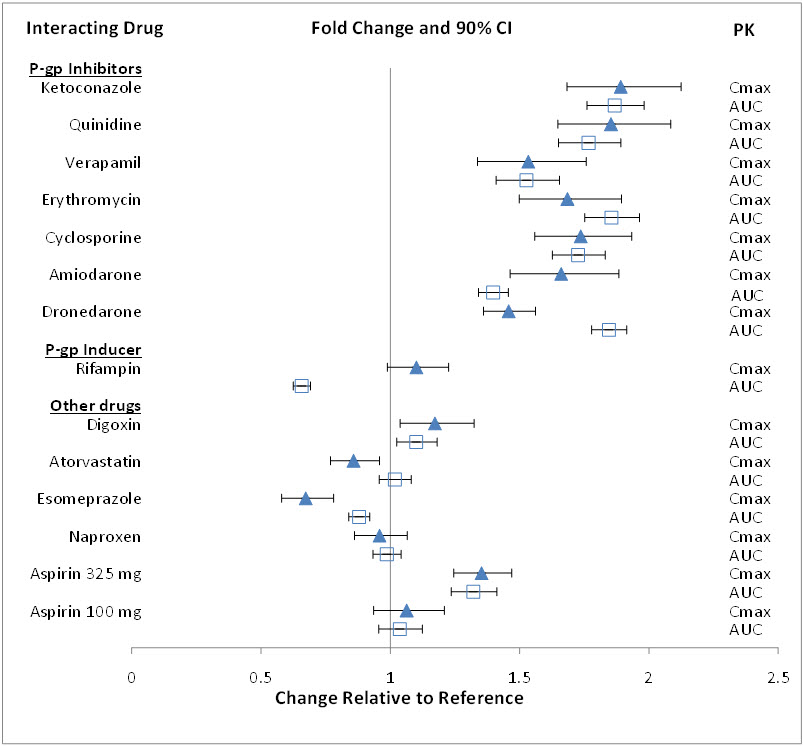
The effect of co-administered amiodarone, cyclosporine, dronedarone, erythromycin, ketoconazole, quinidine, verapamil, and rifampin on edoxaban exposure is shown in Figure 12.1.
Figure 12.1: Summary of Drug Interaction Study Results
Impact of Edoxaban on Other Drugs
Edoxaban increased the Cmax of concomitantly administered digoxin by 28%; however, the AUC was not affected. Edoxaban had no effect on the Cmax and AUC of quinidine.
Edoxaban decreased the Cmax and AUC of concomitantly administered verapamil by 14% and 16%, respectively.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Edoxaban was not carcinogenic when administered daily to mice and rats by oral gavage for up to 104 weeks. The highest dose tested (500 ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Edoxaban was not carcinogenic when administered daily to mice and rats by oral gavage for up to 104 weeks. The highest dose tested (500 mg/kg/day) in male and female mice was 3 and 6 times, respectively, the human exposure (AUC) at the human dose of 60 mg/day, and the highest doses tested in male (600/400 mg/kg/day) and female (200 mg/kg/day) rats were 8 and 14 times, respectively, the human exposure at the human dose of 60 mg/day.
Edoxaban and its human-specific metabolite, M-4, were genotoxic in in vitro chromosomal aberration tests but were not genotoxic in the in vitro bacterial reverse mutation (Ames test), in in vitro human lymphocytes micronucleus test, in in vivo rat bone marrow micronucleus test, in in vivo rat liver micronucleus test, and in in vivo unscheduled DNA synthesis tests.
Edoxaban showed no effects on fertility and early embryonic development in rats at doses of up to 1000 mg/kg/day (162 times the human dose of 60 mg/day normalized to body surface area).
-
14 CLINICAL STUDIES14.1 Nonvalvular Atrial Fibrillation - The ENGAGE AF-TIMI 48 Study - The ENGAGE AF-TIMI 48 (NCT00781391) study was a multi-national, double-blind, non-inferiority study comparing the efficacy ...
14.1 Nonvalvular Atrial Fibrillation
The ENGAGE AF-TIMI 48 Study
The ENGAGE AF-TIMI 48 (NCT00781391) study was a multi-national, double-blind, non-inferiority study comparing the efficacy and safety of two SAVAYSA treatment arms (60 mg and 30 mg) to warfarin (titrated to INR 2.0 to 3.0) in reducing the risk of stroke and systemic embolic events in patients with NVAF. The non-inferiority margin (degree of inferiority of SAVAYSA to warfarin that was to be ruled out) was set at 38%, reflecting the substantial effect of warfarin in reducing strokes. The primary analysis included both ischemic and hemorrhagic strokes.
To enter the study, patients had to have one or more of the following additional risk factors for stroke:
- a prior stroke (ischemic or unknown type), transient ischemic attack (TIA) or non-CNS systemic embolism, or
- 2 or more of the following risk factors:
- –
- age ≥ 75 years,
- –
- hypertension,
- –
- heart failure, or
- –
- diabetes mellitus
A total of 21,105 patients were randomized and followed for a median of 2.8 years and treated for a median of 2.5 years. Patients in the SAVAYSA treatment arms had their dose halved (60 mg halved to 30 mg or 30 mg halved to 15 mg) if one or more of the following clinical factors were present: CrCL ≤ 50 mL/min, low body weight (≤ 60 kg) or concomitant use of specific P-gp inhibitors (verapamil, quinidine, dronedarone). Patients on antiretroviral therapy (ritonavir, nelfinavir, indinavir, saquinavir) as well as cyclosporine were excluded from the study. Approximately 25% of patients in all treatment groups received a reduced dose at baseline, and an additional 7% were dose-reduced during the study. The most common reason for dose reduction was a CrCL ≤ 50 mL/min at randomization (19% of patients).
Patients were well balanced with respect to demographic and baseline characteristics. The percentages of patients age ≥ 75 years and ≥ 80 years were approximately 40% and 17%, respectively. The majority of patients were Caucasian (81%) and male (62%). Approximately 40% of patients had not taken a Vitamin K Antagonist (VKA) (i.e., never took a VKA or had not taken a VKA for more than 2 months).
The mean patient body weight was 84 kg (185 lbs) and 10% of patients had a body weight of ≤ 60 kg. Concomitant diseases of patients in this study included hypertension (94%), congestive heart failure (58%), and prior stroke or transient ischemic attack (28%). At baseline, approximately 30% of patients were on aspirin and approximately 2% of patients were taking a thienopyridine.
Patients randomized to the warfarin arm achieved a mean TTR (time in therapeutic range, INR 2.0 to 3.0) of 65% during the course of the study.
The primary endpoint of the study was the occurrence of first stroke (either ischemic or hemorrhagic) or of a systemic embolic event (SEE) that occurred during treatment or within 3 days from the last dose taken. In the overall results of the study, shown in Table 14.1, both treatment arms of SAVAYSA were non-inferior to warfarin for the primary efficacy endpoint of stroke or SEE. However, the 30 mg (15 mg dose-reduced) treatment arm was numerically less effective than warfarin for the primary endpoint, and was also markedly inferior in reducing the rate of ischemic stroke. Based on the planned superiority analysis (ITT, which required p < 0.01 for success), statistical superiority of the 60 mg (30 mg dose-reduced) treatment arm compared to warfarin was not established in the total study population, but there was a favorable trend [HR (99% CI): 0.87 (0.71, 1.07)].
Table 14.1: Strokes and Systemic Embolic Events in the ENGAGE AF-TIMI 48 Study (mITT, on Treatment*) Events SAVAYSA
30 mg†
(N = 7002)
n (%/yr)‡SAVAYSA
60 mg†
(N = 7012)
n (%/yr)‡Warfarin
(N = 7012)
n (%/yr)‡SAVAYSA 30 mg vs. warfarin
HR (CI)§
p-valueSAVAYSA 60 mg vs. warfarin
HR (CI)§
p-valueAbbreviations: HR = Hazard Ratio versus Warfarin, CI = Confidence Interval, n = number of events, mITT = Modified Intent-to-Treat, N = number of patients in mITT population, SEE = Systemic Embolic Event, yr = year. - *
- Includes events during treatment or within 3 days of stopping study treatment
- †
- Includes patients dose-reduced to 15 mg for the 30 mg treatment group and 30 mg for the 60 mg treatment group
- ‡
- The event rate (%/yr) is calculated as number of events/subject-year exposure.
- §
- 97.5% CI for primary endpoint of First Stroke or SEE. 95% CI for Ischemic Stroke, Hemorrhagic Stroke or Systemic Embolism
First Stroke or SEE 253 (1.6) 182 (1.2) 232 (1.5) 1.07 (0.87, 1.31)
p = 0.440.79 (0.63, 0.99)
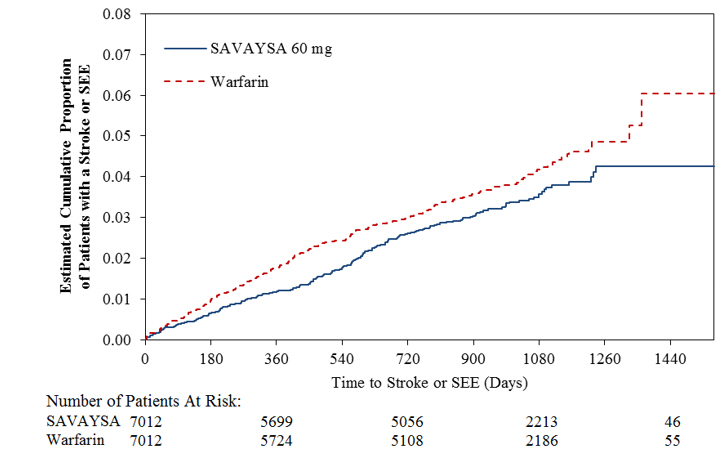
p = 0.017Ischemic Stroke 225 (1.4) 135 (0.9) 144 (0.9) 1.54 (1.25, 1.90) 0.94 (0.75, 1.19) Hemorrhagic Stroke 18 (0.1) 39 (0.3) 75 (0.5) 0.24 (0.14, 0.39) 0.52 (0.36, 0.77) Systemic Embolism 10 (< 0.1) 8 (< 0.1) 13 (< 0.1) 0.75 (0.33, 1.72) 0.62 (0.26, 1.50) Figure 14.1 is a plot of the time from randomization to the occurrence of the first primary endpoint in all patients randomized to 60 mg SAVAYSA or warfarin.
Figure 14.1: Kaplan-Meier Cumulative Event Rate Estimates for Primary Endpoint (first occurrence of stroke or SEE) (mITT*) - *
- On-treatment Study Period defined as Initial Dose to Final Dose + three days
The incidence rate of the primary endpoint of stroke or SEE in patients (N = 1776) treated with the 30 mg reduced dose of SAVAYSA because of a CrCL level ≤ 50 mL/min, low body weight ≤ 60 kg, or the concomitant use of a P-gp inhibitor drug, was 1.79% per year. Patients with any of these characteristics who were randomized to receive warfarin had an incidence rate of the primary endpoint of 2.21% per year [HR (95% CI): 0.81 (0.58, 1.13)].
In all randomized patients during the overall study period, the rates of CV death with SAVAYSA and warfarin were 2.74% per year vs. 3.17% per year, respectively [HR (95% CI): 0.86 (0.77, 0.97)].
The results in the ENGAGE AF-TIMI 48 study for the primary efficacy endpoint for most major subgroups are displayed in Figure 14.2.
Figure 14.2: ENGAGE AF-TIMI 48 Study: Primary Efficacy Endpoint by Subgroups (ITT Analysis Set) Note: The figure above presents effects in various subgroups all of which are baseline characteristics and most of which were pre-specified. The 95% confidence limits that are shown do not take into account how many comparisons were made, nor do they reflect the effect of a particular factor after adjustment for all other factors. Apparent homogeneity or heterogeneity among groups should not be over-interpreted. 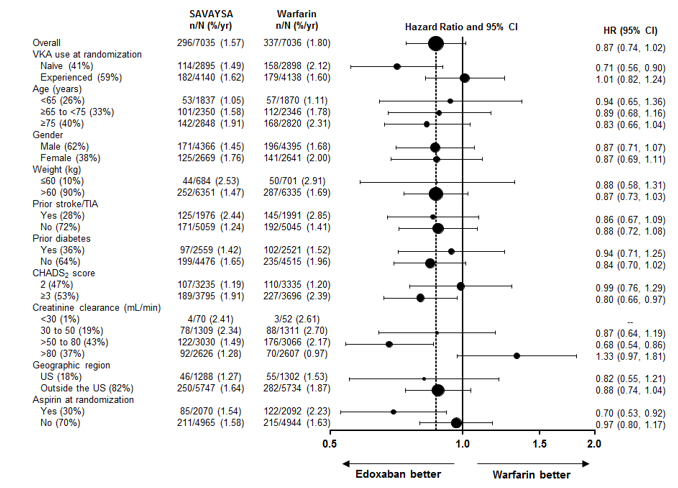
The results of the ENGAGE AF-TIMI 48 study show a strong relationship between the blood levels of edoxaban and its effectiveness in reducing the rate of ischemic stroke. There was a 64% increase in the ischemic stroke rate in patients in the 30 mg treatment arm (including patients with dose reduced to 15 mg) compared to the 60 mg treatment arm (including patients with dose reduced to 30 mg). Approximately half of the SAVAYSA dose is eliminated by the kidney, and edoxaban blood levels are lower in patients with better renal function, averaging about 30% less in patients with CrCL of > 80 mL/min, and 40% less in patients with CrCL > 95 mL/min when compared to patients with a CrCL of > 50 to ≤ 80 mL/min. Given the clear relationship of dose and blood levels to effectiveness in the ENGAGE AF-TIMI 48 study, it could be anticipated that patients with better renal function would show a smaller effect of SAVAYSA compared to warfarin than would patients with mildly impaired renal function, and this was in fact observed.
Table 14.2 shows the results for the study primary efficacy endpoint of first stroke or SEE as well as the effects on ischemic and hemorrhagic stroke in the pre-randomization CrCL subgroups for SAVAYSA 60 mg (including 30 mg dose-reduced) and warfarin. There was a decreased rate of ischemic stroke with SAVAYSA 60 mg compared to warfarin in patients with CrCL > 50 to ≤ 80 mL/min [HR (95% CI): 0.63 (0.44, 0.89)]. In patients with CrCL > 80 to ≤ 95 mL/min the results for ischemic stroke slightly favor warfarin with a confidence interval that crosses 1.0 [HR (95% CI): 1.11 (0.58, 2.12)]. The rate of ischemic stroke was higher relative to warfarin in the patients with CrCL > 95 mL/min [HR (95% CI): 2.16 (1.17, 3.97)]. Pharmacokinetic data indicate that patients with CrCL > 95 mL/min had lower plasma edoxaban levels, along with a lower rate of bleeding relative to warfarin than patients with CrCL ≤ 95 mL/min. Consequently, SAVAYSA should not be used in patients with CrCL > 95 mL/min [see Dosage and Administration (2.1), Warnings and Precautions (5.1), Adverse Reactions (6.1), and Clinical Pharmacology (12.3)].
In patients with CrCL ≤ 95 mL/min, the SAVAYSA 60 mg (30 mg dose-reduced) treatment arm reduced the risk of stroke or SEE when compared to warfarin [HR (95% CI): 0.68 (0.55, 0.84)].
In the indicated population (CrCL ≤ 95 mL/min), during the overall study period, the rates of CV death with SAVAYSA and warfarin were 2.95% per year vs. 3.59% per year, respectively [HR (95% CI): 0.82 (0.72, 0.93)].
Table 14.2: Primary Endpoint, Ischemic and Hemorrhagic Stroke Results in as a Function of Baseline Creatinine Clearance (mITT Population, On-Treatment) STROKE TYPE
Renal Function Subgroups*Treatment Arm n (N) Event Rate
(%/yr)SAVAYSA 60 mg vs. Warfarin HR
(95% CI)Abbreviations: HR = Hazard Ratio versus Warfarin, CI = Confidence Interval, n = number of events, mITT = Modified Intent-to-Treat, N = number of patients in mITT population, yr = year. - *
- Renal function subgroups are based on estimated creatinine clearance in mL/min calculated using the Cockcroft-Gault formula.
- †
- 83% of patients with pre-randomization CrCL ≤ 50 mL/min in the SAVAYSA 60 mg group were dose-reduced and consequently received SAVAYSA 30 mg daily. All patients in the warfarin group with CrCL ≤ 50 mL/min were treated in the same way as those with higher levels of CrCL.
- ‡
- See Boxed Warning
PRIMARY ENDPOINT
(STROKE/SEE)≤ 95 (Indicated Population) Warfarin 211 (5485) 1.8 0.68 (0.55, 0.84) SAVAYSA 60 mg 142 (5417) 1.2 ≤ 50† Warfarin 50 (1356) 2.0 0.90 (0.60, 1.34) SAVAYSA 60 mg 45 (1372) 1.8 > 50 to ≤ 80 Warfarin 135 (3053) 2.0 0.53 (0.40, 0.70) SAVAYSA 60 mg 71 (3020) 1.1 > 80 to ≤ 95 Warfarin 26 (1076) 1.0 1.05 (0.61, 1.82) SAVAYSA 60 mg 26 (1025) 1.1 > 95‡ Warfarin 21 (1527) 0.6 1.87 (1.10, 3.17) SAVAYSA 60 mg 40 (1595) 1.0 ISCHEMIC STROKE ≤ 95 (Indicated Population) Warfarin 129 (5485) 1.1 0.80 (0.62, 1.04) SAVAYSA 60 mg 102 (5417) 0.9 ≤ 50† Warfarin 28 (1356) 1.1 1.11 (0.66, 1.84) SAVAYSA 60 mg 31 (1372) 1.2 > 50 to ≤ 80 Warfarin 83 (3053) 1.2 0.63 (0.44, 0.89) SAVAYSA 60 mg 52 (3020) 0.8 > 80 to ≤ 95 Warfarin 18 (1076) 0.7 1.11 (0.58, 2.12) SAVAYSA 60 mg 19 (1025) 0.8 > 95‡ Warfarin 15 (1527) 0.4 2.16 (1.17, 3.97) SAVAYSA 60 mg 33 (1595) 0.9 HEMORRHAGIC STROKE ≤ 95 (Indicated Population) Warfarin 70 (5485) 0.6 0.50 (0.33, 0.75) SAVAYSA 60 mg 34 (5417) 0.3 ≤ 50† Warfarin 18 (1356) 0.7 0.66 (0.32, 1.36) SAVAYSA 60 mg 12 (1372) 0.5 > 50 to ≤ 80 Warfarin 45 (3053) 0.7 0.38 (0.22, 0.67) SAVAYSA 60 mg 17 (3020) 0.3 > 80 to ≤ 95 Warfarin 7 (1076) 0.3 0.76 (0.24, 2.38) SAVAYSA 60 mg 5 (1025) 0.2 > 95‡ Warfarin 6 (1527) 0.2 0.98 (0.31, 3.05) SAVAYSA 60 mg 6 (1595) 0.2 Transition to Other Anticoagulants in the ENGAGE AF-TIMI 48 Study
In the ENGAGE AF-TIMI 48 study, the schemes for transitioning from study medication to open-label warfarin at the end of study were associated with similar rates of stroke and systemic embolism in the SAVAYSA 60 mg and warfarin groups [see Dosage and Administration (2.4)]. In the SAVAYSA 60 mg group 7 (0.2%) of 4529 patients had a stroke or SEE compared to 7 (0.2%) of 4506 patients in the warfarin arm.
Close14.2 Treatment of Deep Vein Thrombosis and Pulmonary Embolism
SAVAYSA for the treatment of patients with deep vein thrombosis (DVT) and pulmonary embolism (PE) was studied in a multi-national, double-blind study (Hokusai VTE) (NCT00986154) which compared the efficacy and safety of SAVAYSA 60 mg orally once daily to warfarin (titrated to INR 2.0 to 3.0) in patients with acute symptomatic venous thromboembolism (VTE) (DVT or PE with or without DVT). All patients had VTE confirmed by appropriate diagnostic imaging at baseline and received initial heparin therapy with low molecular weight heparin (LMWH) or unfractionated heparin for at least 5 days [median LMWH/heparin treatment in the SAVAYSA 60 mg group was 7 days, and in the warfarin group it was 8.0 days] and until INR (sham or real) was ≥ 2.0 on two measurements. Blinded drug treatment in the warfarin arm was started concurrently with initial heparin therapy and in the SAVAYSA arm after discontinuation of initial heparin. Patients randomized to SAVAYSA received 30 mg once daily if they met one or more of the following criteria: CrCL 30 to 50 mL/min, body weight ≤ 60 kg, or concomitant use of specific P-gp inhibitors (verapamil and quinidine or the short-term concomitant administration of azithromycin, clarithromycin, erythromycin, oral itraconazole or oral ketoconazole). The edoxaban dosage regimen was to be returned to the regular dosage of 60 mg once daily at any time the subject is not taking the concomitant medication provided no other criteria for dose reduction are met. Other P-gp inhibitors were not permitted in the study. Patients on antiretroviral therapy (ritonavir, nelfinavir, indinavir, saquinavir) as well as cyclosporine were excluded from the Hokusai VTE study. The concomitant use of these drugs with SAVAYSA has not been studied in patients. The treatment duration was from 3 months up to 12 months, determined by investigator based on patient clinical features. Patients were excluded if they required thrombectomy, insertion of a caval filter, use of a fibrinolytic agent, or use of other P-gp inhibitors, had a creatinine clearance < 30 mL/min, significant liver disease, or active bleeding. The primary efficacy outcome was symptomatic VTE, defined as the composite of recurrent DVT, new non-fatal symptomatic PE, and fatal PE during the 12-month study period.
A total of 8292 patients were randomized to receive SAVAYSA or warfarin and were followed for a mean treatment duration of 252 days for SAVAYSA and 250 days for warfarin. The mean age was approximately 56 years. The population was 57% male, 70% Caucasian, 21% Asian, and about 4% Black. The presenting diagnosis was PE (with or without DVT) in 40.7% and DVT only in 59.3% of patients. At baseline, 27.6% of patients had temporary risk factors only (e.g., trauma, surgery, immobilization, estrogen therapy). Overall 9.4% had a history of cancer, 17.3% of the patients had an age ≥ 75 years and/or a body weight ≤ 50 kg, and/or a CrCL < 50 mL/min, and 31.4% of patients had NT-ProBNP ≥ 500 pg/mL.
Aspirin was taken as on treatment concomitant antithrombotic medication by approximately 9% of patients in both groups.
In the warfarin group, the median TTR (time in therapeutic range, INR 2.0 to 3.0) was 65.6%.
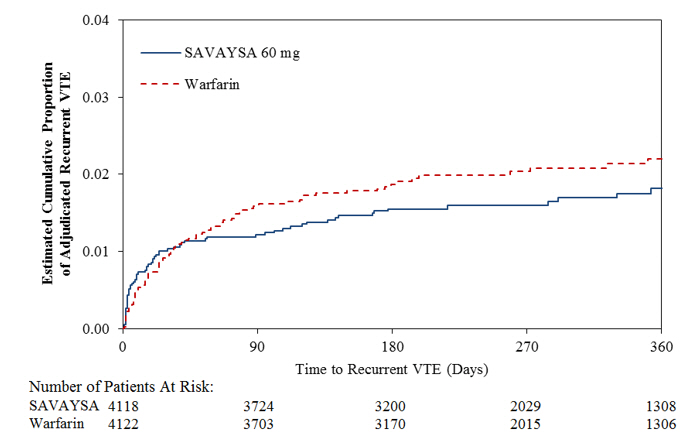
A total of 8240 patients (n = 4118 for SAVAYSA and n = 4122 for warfarin) received study drug and were included in the modified intent-to-treat (mITT) population. SAVAYSA was demonstrated to be non-inferior to warfarin for the primary endpoint of recurrent VTE [HR (95% CI): 0.89 (0.70, 1.13)] (Table 14.3, Figure 14.3).
Table 14.3: Primary Composite Efficacy Endpoint Results in Hokusai VTE (mITT Overall Study Period) Primary Endpoint SAVAYSA*
n/N (%)Warfarin
n/N (%)SAVAYSA vs. Warfarin
HR (95% CI)Abbreviations: mITT = modified intent-to-treat; HR =hazard ratio vs. warfarin; CI =confidence interval; N = number of patients in mITT population; n = number of events - *
- Includes patients dose-reduced to 30 mg. Among the 1452 (17.6%) patients with low body weight (≤ 60 kg), moderate renal impairment (CrCL ≤ 50 mL/min), or concomitant use of P-gp inhibitors in the Hokusai VTE study, 22 (3.0%) of the SAVAYSA patients (30 mg once daily, n = 733) and 30 (4.2%) of warfarin patients (n = 719) had a symptomatic recurrent VTE event
- †
- Primary Efficacy Endpoint: Symptomatic recurrent VTE (i.e., the composite endpoint of DVT, non-fatal PE and fatal PE)
- ‡
- Index PE refers to patients whose presenting diagnosis was PE (with or without concomitant DVT)
- §
- Index DVT refers to patients whose presenting diagnosis was DVT only
All patients with symptomatic recurrent VTE† 130/4118 (3.2) 146/4122 (3.5) 0.89 (0.70,1.13) PE with or without DVT 73/4118 (1.8) 83/4122 (2.0) - Fatal PE and Death where PE cannot be ruled out 24/4118 (0.6) 24/4122 (0.6) - Non-fatal PE 49/4118 (1.2) 59/4122 (1.4) - DVT only 57/4118 (1.4) 63/4122 (1.5) - Index PE‡ patients with symptomatic recurrent VTE 47/1650 (2.8) 65/1669 (3.9) - Index DVT§ patients with symptomatic recurrent VTE 83/2468 (3.4) 81/2453 (3.3) - Figure 14.3: Kaplan-Meier Cumulative Event Rate Estimates for Adjudicated Recurrent VTE (mITT analysis – on treatment)
The Hokusai VTE Cancer Study
In the Hokusai VTE Cancer study (NCT02073682), 1050 patients were randomized to receive SAVAYSA 60 mg once daily [30 mg dose reduced per the dose adjustment regimen used in Engage AF-TIMI 48 and Hokusai VTE studies, (see The Hokusai VTE Study)] after at least 5 days of low-molecular-weight heparin treatment or dalteparin (200 IU/kg day 1-30; 150 IU/kg day 31 to the end of treatment). The treatment duration was for a minimum of 6 months and up to 12 months.
The efficacy of SAVAYSA was based upon the rate of recurrent VTE (mITT) during the overall study period. SAVAYSA was non-inferior to dalteparin for the rate of recurrent VTE. Recurrent VTE occurred in 7.9% (41/522) and 11.3% (59/524) of patients in the SAVAYSA and dalteparin groups, respectively [HR (95% CI): 0.71 (0.48, 1.06)].
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSAVAYSA (edoxaban) is supplied as round shaped, film-coated, non-scored tablets containing edoxaban tosylate equivalent to 60, 30 or 15 mg of SAVAYSA, packaged in bottles and ...
SAVAYSA (edoxaban) is supplied as round shaped, film-coated, non-scored tablets containing edoxaban tosylate equivalent to 60, 30 or 15 mg of SAVAYSA, packaged in bottles and blisters.
NDC 65597-xxx-yy Strength Color Deboss yy xxx Bottle of Blister of 30 90 500 10 × 10* 10 × 5† 15 mg orange DSC L15 201 30 - - - - 30 mg pink DSC L30 202 30 90 50 10 05 60 mg yellow DSC L60 203 30 90 50 10 05 CloseStore at 20-25°C (68-77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature].
Keep out of the reach of children.
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Advise patients of the following: Instructions for Patient Use - Advise patients to take SAVAYSA exactly as ...
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Advise patients of the following:
Instructions for Patient Use
- Advise patients to take SAVAYSA exactly as prescribed.
- Remind patients to not discontinue SAVAYSA without talking to the healthcare provider who prescribed it.
- Instruct patients to keep an adequate supply of tablets to ensure continuous dosing of SAVAYSA.
- Instruct patients who cannot swallow the tablet whole to crush SAVAYSA, combine with 2 to 3 ounces of water or applesauce and ingest immediately.
- Instruct patients who require a gastric tube to crush the SAVAYSA tablet and mix it with 2 to 3 ounces of water before administering immediately via the gastric feeding tube.
- Inform patients that if a dose is missed, they should take SAVAYSA as soon as possible the same day, and resume the normal dosing schedule the following day. The dose should not be doubled to make up for a missing dose.
Bleeding Risk
- Advise patients that they may bleed more easily, may bleed longer, or bruise more easily when treated with SAVAYSA.
- Instruct patients to report any unusual bleeding immediately to their healthcare provider.
- For patients that are having neuraxial anesthesia or spinal puncture, advise patients to watch for signs and symptoms of spinal or epidural hematoma, such as back pain, tingling, numbness (especially in the lower limbs), muscle weakness, and stool or urine incontinence. If any of these symptoms occur, advise the patient to contact his or her physician immediately [see Boxed Warning].
Invasive or Surgical Procedures
- Remind patients to inform their healthcare providers that they are taking SAVAYSA before any surgery, medical, or dental procedure is scheduled.
Concomitant Medication and Herbals
- Remind patients to inform their healthcare providers and dentists if they plan to take, or are taking any prescription medications, over-the-counter drugs or herbal products.
ClosePregnancy
- Remind patients to inform their healthcare provider immediately if they become pregnant or intend to become pregnant during treatment with SAVAYSA.
- Inform patients to not breastfeed if they are taking SAVAYSA [see Use in Specific Populations (8.2)].
-
SPL UNCLASSIFIED SECTIONManufactured by: Daiichi Sankyo Co., LTD. Tokyo 103-8426 - Japan - Distributed by: Daiichi Sankyo, Inc. Basking Ridge, NJ 07920-2311 USA - SAVAYSA® is a trademark of Daiichi Sankyo Co. ...
Manufactured by:
Daiichi Sankyo Co., LTD.
Tokyo 103-8426
JapanDistributed by:
Daiichi Sankyo, Inc.
Basking Ridge, NJ 07920-2311 USA
SAVAYSA® is a trademark of Daiichi Sankyo Co., LTD.
Copyright © 2023, Daiichi Sankyo, Inc.USPI-SAV-C9-1023-r104
Close -
MEDICATION GUIDEMEDICATION GUIDE - SAVAYSA (sa vaye' sah) (edoxaban) tablets - This Medication Guide has been approved by the U.S. Food and Drug Administration.Revised: 10/2023 What is the most ...Close
MEDICATION GUIDE
SAVAYSA (sa vaye' sah)
(edoxaban) tabletsThis Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 10/2023 What is the most important information I should know about SAVAYSA? -
For people who take SAVAYSA for nonvalvular atrial fibrillation (a type of irregular heartbeat):
People with nonvalvular atrial fibrillation are at an increased risk of forming a blood clot in the heart, which can travel to the brain, causing a stroke, or to other parts of the body. SAVAYSA reduces your risk of having a stroke by helping to prevent clots from forming.- Your healthcare provider should check your kidney function before you start taking SAVAYSA. People whose kidneys work really well should not receive SAVAYSA because it may not work well to prevent stroke.
- Do not stop taking SAVAYSA without first talking to the healthcare provider who prescribed it for you. Stopping SAVAYSA increases your risk of having a stroke.
-
SAVAYSA can cause bleeding which can be serious, and sometimes lead to death. This is because SAVAYSA is a blood thinner medicine that reduces blood clotting. During treatment with SAVAYSA, you may bleed more easily, bleed longer, or bruise more easily. Call your healthcare provider or get medical help right away if you experience bleeding that is severe (for example, coughing up or vomiting blood) or bleeding that cannot be controlled.
You may have a higher risk of bleeding if you take SAVAYSA and take other medicines that increase your risk of bleeding, including:- aspirin or aspirin-containing products
- long-term (chronic) use of nonsteroidal anti-inflammatory drugs (NSAIDs)
- long-term (chronic) use of blood thinner medicines, such as:
- warfarin sodium (Coumadin, Jantoven)
- any medicine that contains heparin
- selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs)
- other medicines to prevent or treat blood clots
-
Spinal or epidural blood clots (hematoma). People who take a blood thinner medicine (anticoagulant) like SAVAYSA, and have medicine injected into their spinal and epidural area, or have a spinal puncture have a risk of forming a blood clot that can cause long-term or permanent loss of the ability to move (paralysis). Your risk of developing a spinal or epidural blood clot is higher if:
- a thin tube called an epidural catheter is placed in your back to give you certain medicine
- you take NSAIDs or a medicine to prevent blood from clotting
- you have a history of difficult or repeated epidural or spinal punctures
- you have a history of problems with your spine or have had surgery on your spine.
- SAVAYSA is not for people with mechanical heart valves or people who have moderate to severe narrowing (stenosis) of their mitral valve.
- SAVAYSA is not for use in people with antiphospholipid syndrome (APS), especially with positive triple antibody testing, who have a history of blood clots.
What is SAVAYSA?
SAVAYSA is a prescription medicine used to:- reduce the risk of stroke and blood clots in people who have atrial fibrillation not caused by a heart valve problem (nonvalvular atrial fibrillation).
- treat blood clots in the veins of your legs (deep vein thrombosis) or lungs (pulmonary embolism), after you have been treated with an injectable blood thinner medicine for 5 to 10 days.
Who should not take SAVAYSA?
Do not take SAVAYSA if you currently have certain types of abnormal bleeding.Before taking SAVAYSA, tell your healthcare provider about all of your medical conditions, including if you: - have liver or kidney problems
- have ever had bleeding problems
- have a mechanical heart valve
- have antiphospholipid syndrome
- have cancer of the stomach or intestine (gastrointestinal cancer)
- are pregnant or plan to become pregnant. It is not known if SAVAYSA will harm your unborn baby. Tell your healthcare provider right away if you become pregnant during treatment with SAVAYSA.
Females who are able to become pregnant: Talk with your healthcare provider about pregnancy planning during treatment with SAVAYSA. Talk with your healthcare provider about your risk for severe uterine bleeding if you are treated with blood thinner medicines, including SAVAYSA. - are breastfeeding or plan to breastfeed. It is not known if SAVAYSA passes into your breast milk. Do not breastfeed during treatment with SAVAYSA.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some of your other medicines may affect the way SAVAYSA works. Certain medicines may increase your risk of bleeding or stroke when taken with SAVAYSA. See "What is the most important information I should know about SAVAYSA?"
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.How should I take SAVAYSA? - Take SAVAYSA exactly as prescribed by your healthcare provider.
- Your healthcare provider will decide how long you should take SAVAYSA. Do not change your dose or stop taking SAVAYSA unless your healthcare provider tells you to. If you are taking SAVAYSA for nonvalvular atrial fibrillation, stopping SAVAYSA may increase your risk of having a stroke.
- Take SAVAYSA with or without food.
- If you have difficulty swallowing the tablet whole, talk to your healthcare provider about other ways to take SAVAYSA.
- If you miss a dose of SAVAYSA, take it as soon as you remember on the same day. Take your next dose at your usual time the next day. Do not take more than one dose of SAVAYSA at the same time to make up for a missed dose.
- Do not run out of SAVAYSA. Refill your prescription before you run out. If you take too much SAVAYSA, go to the nearest hospital emergency room or call your healthcare provider right away.
- Call your healthcare provider right away if you fall or injure yourself, especially if you hit your head. Your healthcare provider may need to check you.
What are the possible side effects of SAVAYSA?
SAVAYSA can cause serious side effects.
See "What is the most important information I should know about SAVAYSA?"
The most common side effects in people who take SAVAYSA for nonvalvular atrial fibrillation include bleeding and low red blood cell count (anemia).
The most common side effects in people who take SAVAYSA for deep vein thrombosis and pulmonary embolism include bleeding, rash, abnormal liver function tests, and low red blood cell count (anemia).
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store SAVAYSA? - Store SAVAYSA at room temperature between 68°F to 77°F (20°C to 25°C).
General information about the safe and effective use of SAVAYSA
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use SAVAYSA for a condition for which it was not prescribed. Do not give SAVAYSA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about SAVAYSA that is written for health professionals.What are the ingredients in SAVAYSA?
Active ingredient: edoxaban tosylate monohydrate
Inactive ingredients: mannitol, pregelatinized starch, crospovidone, hydroxypropyl cellulose, magnesium stearate, talc, and carnauba wax. The color coatings contain hypromellose, titanium dioxide, talc, polyethylene glycol 8000, iron oxide yellow (60 mg tablets and 15 mg tablets), and iron oxide red (30 mg tablets and 15 mg tablets).
Manufactured by: Daiichi Sankyo Co., LTD., Tokyo 103-8426, Japan
Distributed by: Daiichi Sankyo, Inc., Basking Ridge, NJ 07920-2311 USA
The brands listed above are trademarks of their respective owners.
Copyright© 2023, Daiichi Sankyo, Inc.
USMG-SAV-C9-1023-r103
For more information, call 1-877-437-7763 or go to https://savaysa.com. -
For people who take SAVAYSA for nonvalvular atrial fibrillation (a type of irregular heartbeat):
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - NDC 65597-201-30 - Savaysa - (edoxaban) tablets - 15 mg - 30 TABLETS - Rx Only - PRINCIPAL DISPLAY PANEL - NDC 65597-202-30 - Savaysa - (edoxaban) tablets - 30 mg - 30 TABLETS - Rx Only
PRINCIPAL DISPLAY PANEL
NDC 65597-201-30
Savaysa
(edoxaban) tablets
15 mg
30 TABLETS
Rx Only
PRINCIPAL DISPLAY PANEL
NDC 65597-202-30
Savaysa
(edoxaban) tablets
30 mg
30 TABLETS
Rx Only
Close
-
PRINCIPAL DISPLAY PANEL - 30 mg Blister Pack CartonNDC 65597-202-05 - Package contains 5 blister packs - of 10 tablets each. Savaysa® (edoxaban) tablets - 30 mg - Dispense with medication guide - For Hospital Use Only - Daiichi-Sankyo - Rx only
NDC 65597-202-05
Package contains 5 blister packs
of 10 tablets each.Savaysa®
(edoxaban) tablets30 mg
Dispense with medication guide
For Hospital Use OnlyDaiichi-Sankyo
Rx only
Close
-
PRINCIPAL DISPLAY PANEL - 60 mg Blister Pack CartonNDC 65597-203-05 - Package contains 5 blister packs - of 10 tablets each. Savaysa® (edoxaban) tablets - 60 mg - Dispense with medication guide - For Hospital Use Only - Daiichi-Sankyo - Rx only
NDC 65597-203-05
Package contains 5 blister packs
of 10 tablets each.Savaysa®
(edoxaban) tablets60 mg
Dispense with medication guide
For Hospital Use OnlyDaiichi-Sankyo
Rx only
Close
-
INGREDIENTS AND APPEARANCEProduct Information
SAVAYSA edoxaban tosylate tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65597-201 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EDOXABAN TOSYLATE (UNII: 32W99UE810) (EDOXABAN - UNII:NDU3J18APO) EDOXABAN 15 mg Product Characteristics Color ORANGE Score no score Shape ROUND Size 7mm Flavor Imprint Code DSC;L15 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65597-201-07 7 in 1 BOTTLE; Type 0: Not a Combination Product 01/31/2015 2 NDC:65597-201-10 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 01/31/2015 3 NDC:65597-201-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/31/2015 4 NDC:65597-201-50 500 in 1 BOTTLE; Type 0: Not a Combination Product 01/31/2015 5 NDC:65597-201-70 7 in 1 BLISTER PACK; Type 0: Not a Combination Product 01/31/2015 6 NDC:65597-201-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/31/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206316 01/12/2015 SAVAYSA edoxaban tosylate tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65597-202 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EDOXABAN TOSYLATE (UNII: 32W99UE810) (EDOXABAN - UNII:NDU3J18APO) EDOXABAN 30 mg Product Characteristics Color PINK Score no score Shape ROUND Size 9mm Flavor Imprint Code DSC;L30 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65597-202-10 100 in 1 CARTON 01/31/2015 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:65597-202-05 50 in 1 CARTON 01/31/2015 2 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC:65597-202-07 7 in 1 BOTTLE; Type 0: Not a Combination Product 01/31/2015 4 NDC:65597-202-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/31/2015 5 NDC:65597-202-50 500 in 1 BOTTLE; Type 0: Not a Combination Product 01/31/2015 6 NDC:65597-202-70 7 in 1 BLISTER PACK; Type 0: Not a Combination Product 01/31/2015 7 NDC:65597-202-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/31/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206316 01/12/2015 SAVAYSA edoxaban tosylate tablet, film coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65597-203 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EDOXABAN TOSYLATE (UNII: 32W99UE810) (EDOXABAN - UNII:NDU3J18APO) EDOXABAN 60 mg Product Characteristics Color YELLOW Score no score Shape ROUND Size 11mm Flavor Imprint Code DSC;L60 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65597-203-10 100 in 1 CARTON 01/31/2015 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:65597-203-05 50 in 1 CARTON 01/31/2015 2 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC:65597-203-07 7 in 1 BOTTLE; Type 0: Not a Combination Product 01/31/2015 4 NDC:65597-203-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 01/31/2015 5 NDC:65597-203-50 500 in 1 BOTTLE; Type 0: Not a Combination Product 01/31/2015 6 NDC:65597-203-70 7 in 1 BLISTER PACK; Type 0: Not a Combination Product 01/31/2015 7 NDC:65597-203-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 01/31/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA206316 01/12/2015
CloseLabeler - Daiichi Sankyo Inc. (068605067)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
SAVAYSA- edoxaban tosylate tablet, film coated
Number of versions: 18
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Feb 7, 2024 | 26 (current) | download |
| Oct 31, 2023 | 25 | download |
| Sep 21, 2023 | 24 | download |
| Jan 30, 2023 | 22 | download |
| Nov 16, 2022 | 21 | download |
| Oct 8, 2021 | 19 | download |
| Mar 25, 2021 | 17 | download |
| Apr 30, 2020 | 16 | download |
| Aug 28, 2019 | 15 | download |
| Aug 28, 2018 | 14 | download |
| Nov 7, 2017 | 12 | download |
| Oct 13, 2017 | 11 | download |
| Sep 29, 2017 | 10 | download |
| Sep 30, 2016 | 8 | download |
| Sep 27, 2016 | 7 | download |
| Sep 21, 2015 | 6 | download |
| May 19, 2015 | 3 | download |
| Jan 20, 2015 | 2 | download |
RxNorm
SAVAYSA- edoxaban tosylate tablet, film coated
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 1599543 | edoxaban 15 MG Oral Tablet | PSN |
| 2 | 1599543 | edoxaban 15 MG Oral Tablet | SCD |
| 3 | 1599543 | edoxaban 15 MG (as edoxaban tosylate 20.2 MG) Oral Tablet | SY |
| 4 | 1599549 | Savaysa 15 MG Oral Tablet | PSN |
| 5 | 1599549 | edoxaban 15 MG Oral Tablet [Savaysa] | SBD |
| 6 | 1599549 | Savaysa 15 MG (as edoxaban tosylate 20.2 MG) Oral Tablet | SY |
| 7 | 1599549 | Savaysa 15 MG Oral Tablet | SY |
| 8 | 1599551 | edoxaban 30 MG Oral Tablet | PSN |
| 9 | 1599551 | edoxaban 30 MG Oral Tablet | SCD |
| 10 | 1599551 | edoxaban 30 MG (as edoxaban tosylate 40.41 MG) Oral Tablet | SY |
| 11 | 1599553 | Savaysa 30 MG Oral Tablet | PSN |
| 12 | 1599553 | edoxaban 30 MG Oral Tablet [Savaysa] | SBD |
| 13 | 1599553 | Savaysa 30 MG (as edoxaban tosylate 40.41 MG) Oral Tablet | SY |
| 14 | 1599553 | Savaysa 30 MG Oral Tablet | SY |
| 15 | 1599555 | edoxaban 60 MG Oral Tablet | PSN |
| 16 | 1599555 | edoxaban 60 MG Oral Tablet | SCD |
| 17 | 1599555 | edoxaban 60 MG (as edoxaban tosylate 80.82 MG) Oral Tablet | SY |
| 18 | 1599557 | Savaysa 60 MG Oral Tablet | PSN |
| 19 | 1599557 | edoxaban 60 MG Oral Tablet [Savaysa] | SBD |
| 20 | 1599557 | Savaysa 60 MG (as edoxaban tosylate 80.82 MG) Oral Tablet | SY |
| 21 | 1599557 | Savaysa 60 MG Oral Tablet | SY |
Get Label RSS Feed for this Drug
SAVAYSA- edoxaban tosylate tablet, film coated
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=e77d3400-56ad-11e3-949a-0800200c9a66
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
SAVAYSA- edoxaban tosylate tablet, film coated
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 65597-201-07 |
| 2 | 65597-201-10 |
| 3 | 65597-201-30 |
| 4 | 65597-201-50 |
| 5 | 65597-201-70 |
| 6 | 65597-201-90 |
| 7 | 65597-202-05 |
| 8 | 65597-202-07 |
| 9 | 65597-202-10 |
| 10 | 65597-202-30 |
| 11 | 65597-202-50 |
| 12 | 65597-202-70 |
| 13 | 65597-202-90 |
| 14 | 65597-203-05 |
| 15 | 65597-203-07 |
| 16 | 65597-203-10 |
| 17 | 65597-203-30 |
| 18 | 65597-203-50 |
| 19 | 65597-203-70 |
| 20 | 65597-203-90 |