Label: SOTYKTU- deucravacitinib tablet, film coated
- NDC Code(s): 0003-0895-11, 0003-0895-91
- Packager: E.R. Squibb & Sons, L.L.C.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated September 9, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SOTYKTU safely and effectively. See full prescribing information for SOTYKTU. SOTYKTU™ (deucravacitinib) tablets, for oral ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE SOTYKTU™ is indicated for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy. Limitations of Use: SOTYKTU is not recommended ...
-
2 DOSAGE AND ADMINISTRATION 2.1 Recommended Evaluations and Immunizations Prior to Treatment Initiation - Evaluate patients for active and latent tuberculosis (TB) infection prior to initiating treatment with SOTYKTU. If ...
-
3 DOSAGE FORMS AND STRENGTHS Tablets: 6 mg, pink, round, biconvex, laser printed with “BMS 895” and “6 mg” on one side with no content on the other side.
-
4 CONTRAINDICATIONS SOTYKTU is contraindicated in patients with a history of hypersensitivity reaction to deucravacitinib or to any of the excipients in SOTYKTU [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS 5.1 Hypersensitivity - Hypersensitivity reactions such as angioedema have been reported in subjects receiving SOTYKTU. If a clinically significant hypersensitivity reaction occurs, institute ...
-
6 ADVERSE REACTIONS The following adverse reactions are discussed in greater detail in other sections of labeling: • Infections [see Warnings and Precautions (5.2)] • Malignancy including lymphomas [see Warnings ...
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Risk Summary - Available data from case reports on SOTYKTU use during pregnancy are insufficient to evaluate a drug-associated risk of major birth defects ...
-
10 OVERDOSAGE There is no experience regarding human overdosage with SOTYKTU. In case of overdose, consider contacting the Poison Help line (1-800-222-1222) for additional overdosage management ...
-
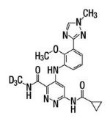
11 DESCRIPTION Deucravacitinib is a tyrosine kinase 2 (TYK2) inhibitor and is described chemically as ...
-
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - Deucravacitinib is an inhibitor of tyrosine kinase 2 (TYK2). TYK2 is a member of the Janus kinase (JAK) family. Deucravacitinib binds to the regulatory domain of TYK2 ...
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - The carcinogenic potential of deucravacitinib was assessed in 2-year rat and 6-month rasH2 transgenic mouse studies. No evidence ...
-
14 CLINICAL STUDIES 14.1 Plaque Psoriasis - The efficacy and safety of SOTYKTU 6 mg once daily were assessed in two multicenter, randomized, double-blind, placebo- and active-controlled clinical trials, PSO-1 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplied - SOTYKTU™ (deucravacitinib) tablets are available as listed in the table below: Tablet Strength - Tablet Color/Shape - Tablet Markings - Package Size - NDC ...
-
17 PATIENT COUNSELING INFORMATION Advise the patient to read the FDA-approved patient labeling (Medication Guide) before starting SOTYKTU therapy and each time the prescription is renewed, as there may be new information they need ...
-
MEDICATION GUIDE - SOTYKTU™ (soh-tik-too) (deucravacitinib) tablets - What is the most important information I should know about SOTYKTU? SOTYKTU may cause serious side ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL NDC 0003-0895-11 - 30 Tablets - SOTYKTUTM - (deucravacitinib) tablets - 6 mg - Rx only - Bristol Myers Squibb
-
INGREDIENTS AND APPEARANCEProduct Information