Label: DENGVAXIA- dengue tetravalent vaccine, live kit
- NDC Code(s): 49281-549-58, 49281-605-01, 49281-606-58
- Packager: Sanofi Pasteur Inc.
- Category: VACCINE LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated October 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DENGVAXIA safely and effectively. See full prescribing information for DENGVAXIA. DENGVAXIA (Dengue Tetravalent Vaccine, Live ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEDENGVAXIA® (Dengue Tetravalent Vaccine, Live) is a vaccine indicated for the prevention of dengue disease caused by dengue virus serotypes 1, 2, 3, and 4. DENGVAXIA is approved for use in ...
-
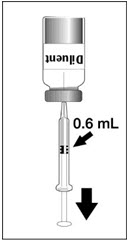
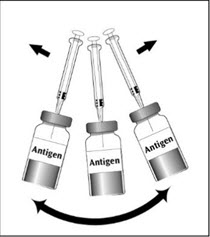
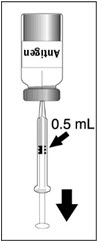
2 DOSAGE AND ADMINISTRATIONFor subcutaneous use only. 2.1 Dose - Three doses (0.5 mL each) 6 months apart (at month 0, 6, and 12). 2.2 Preparation - The package contains a vial of lyophilized vaccine antigen and a vial ...
-
3 DOSAGE FORMS AND STRENGTHSDENGVAXIA is a suspension for injection (supplied as a lyophilized powder to be reconstituted with the supplied diluent, 0.4% NaCl). A single dose, after reconstitution, is 0.5 mL.
-
4 CONTRAINDICATIONS4.1 Hypersensitivity - Do not administer DENGVAXIA to individuals with a history of severe allergic reaction to a previous dose of DENGVAXIA or to any component of DENGVAXIA. [See Description ...
-
5 WARNINGS AND PRECAUTIONS5.1 Increased Risk of Severe Dengue Disease Following DENGVAXIA in Persons Younger than 6 Years of Age Regardless of Previous Infection with Dengue Virus - DENGVAXIA is not approved for use in ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Immunosuppressive Treatments - Immunosuppressive therapies, including irradiation, antimetabolites, alkylating agents, cytotoxic drugs and corticosteroids (used in greater than physiologic ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to DENGVAXIA during pregnancy. Women who receive ...
-
11 DESCRIPTIONDENGVAXIA (Dengue Tetravalent Vaccine, Live) is a sterile suspension for subcutaneous injection. DENGVAXIA is supplied as a vial of lyophilized vaccine antigen, which must be reconstituted at the ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Following administration, DENGVAXIA elicits dengue-specific immune responses against the four dengue virus serotypes. The exact mechanism of protection has not been ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - DENGVAXIA has not been evaluated for carcinogenic or mutagenic potential or impairment of male fertility. Exposure of female rabbits to ...
-
14 CLINICAL STUDIES14.1 Efficacy - The efficacy of DENGVAXIA was evaluated in two randomized, observer-blind, placebo-controlled, multi-center studies. Study 1 (N=20,869) was conducted in individuals 9 through 16 ...
-
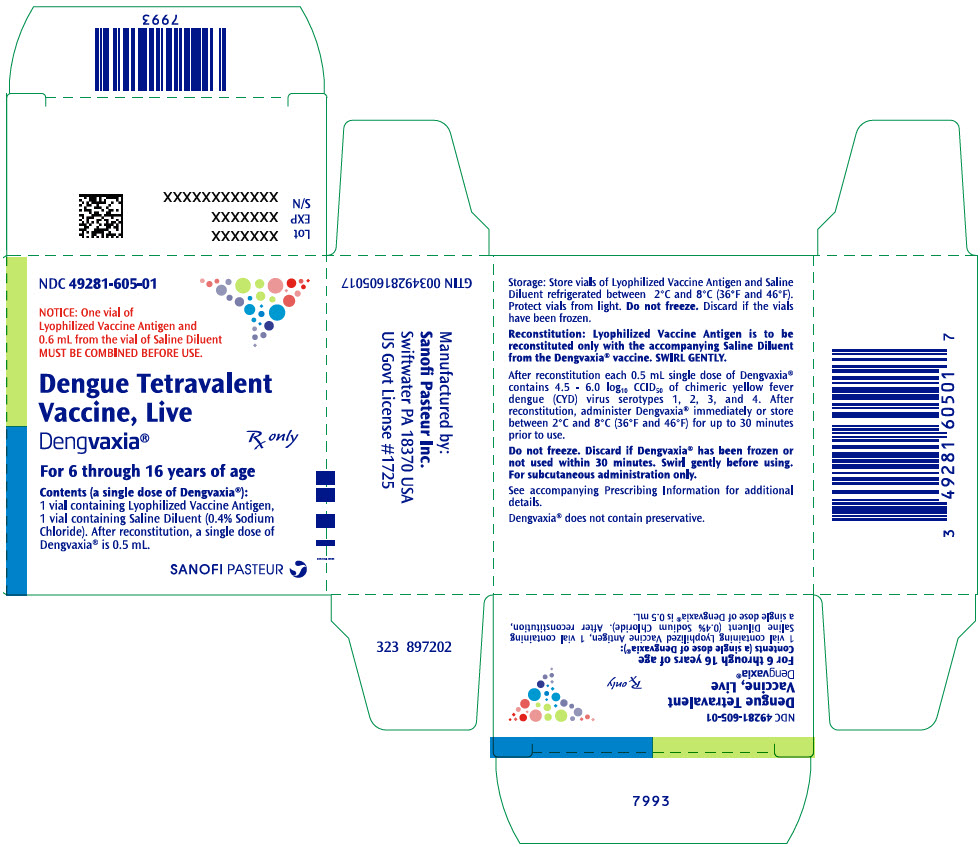
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - An outer package of 1 dose (NDC 49281-605-01) contains 1 single dose vial of Lyophilized Vaccine Antigen (NDC 49281-606-58) and 1 single dose vial of Saline Diluent (NDC ...
-
17 PATIENT COUNSELING INFORMATIONEducate vaccine recipients regarding the most common adverse reactions that occur within 14 days following administration of DENGVAXIA (headache, injection site pain, malaise, asthenia, and ...
-
SPL UNCLASSIFIED SECTIONManufactured and distributed by: Sanofi Pasteur Inc. Swiftwater PA 18370 USA - DENGVAXIA® is a registered trademark of Sanofi, its affiliates and/or its subsidiaries. Adacel® is a registered ...
-
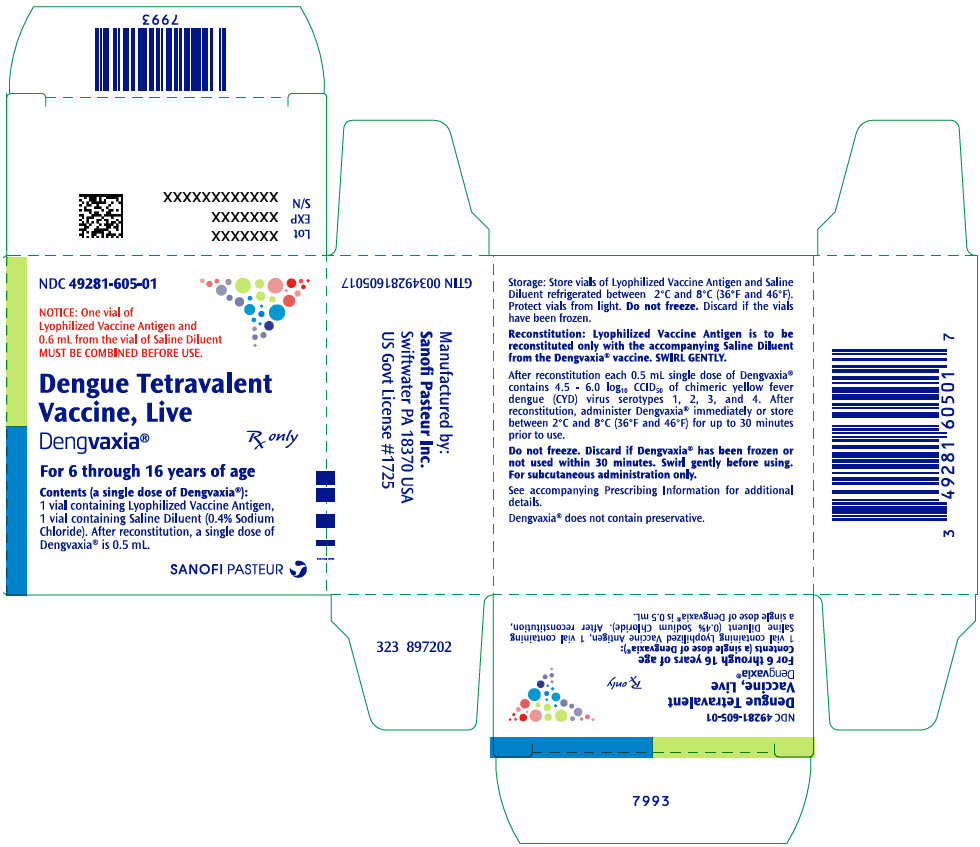
PRINCIPAL DISPLAY PANEL - Kit CartonNDC 49281-605-01 - NOTICE: One vial of - Lyophilized Vaccine Antigen and - 0.6 mL from the vial of Saline Diluent - MUST BE COMBINED BEFORE USE. Dengue Tetravalent - Vaccine, Live - Dengvaxia® Rx only - For 6 ...
-
PRINCIPAL DISPLAY PANEL - 0.5 mL Vial LabelLyophilized Vaccine Antigen - Reconstitute with Saline - Diluent (0.4% NaCl) to form - Dengvaxia® 0.5 mL Single Dose after Reconstitution - Subcutaneous use - Dengvaxia® NDC 49281-606-58 - Rx only - Mfd ...
-
PRINCIPAL DISPLAY PANEL - 0.6 mL Vial LabelSaline Diluent - (0.4% Sodium Chloride) NOT TO BE USED ALONE - Rx only - Add to 1 vial of - Lyophilized Vaccine - Antigen - to form Dengvaxia®
-
INGREDIENTS AND APPEARANCEProduct Information