Label: TECARTUS- brexucabtagene autoleucel suspension
- NDC Code(s): 71287-219-01, 71287-219-02, 71287-220-01, 71287-220-02
- Packager: Kite Pharma, Inc.
- Category: CELLULAR THERAPY
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated June 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TECARTUS safely and effectively. See full prescribing information for TECARTUS. TECARTUS® (brexucabtagene autoleucel) suspension ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: CYTOKINE RELEASE SYNDROME, NEUROLOGIC TOXICITIES, and SECONDARY HEMATOLOGICAL MALIGNANCIES
- Cytokine Release Syndrome (CRS), including life-threatening reactions, occurred in patients receiving TECARTUS. Do not administer TECARTUS to patients with active infection or inflammatory disorders. Treat severe or life-threatening CRS with tocilizumab or tocilizumab and corticosteroids [see Dosage and Administration (2.2, 2.3), Warnings and Precautions (5.1)].
- Neurologic toxicities, including life-threatening reactions, occurred in patients receiving TECARTUS, including concurrently with CRS or after CRS resolution. Monitor for neurologic toxicities after treatment with TECARTUS. Provide supportive care and/or corticosteroids, as needed [see Dosage and Administration (2.2, 2.3), Warnings and Precautions (5.2)].
- T cell malignancies have occurred following treatment of hematologic malignancies with BCMA- and CD19-directed genetically modified autologous T cell immunotherapies [see Warnings and Precautions (5.9)].
- TECARTUS is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS) called the YESCARTA and TECARTUS REMS [see Warnings and Precautions (5.3)].
-
1 INDICATIONS AND USAGETECARTUS is a CD19-directed genetically modified autologous T cell immunotherapy indicated for the treatment of: 1.1 Mantle Cell Lymphoma - Adult patients with relapsed or refractory mantle cell ...
-
2 DOSAGE AND ADMINISTRATIONFor autologous use only. For intravenous use only. Each single infusion bag of TECARTUS contains a suspension of chimeric antigen receptor (CAR)-positive T cells in approximately 68 mL. 2.1 ...
-
3 DOSAGE FORMS AND STRENGTHSTECARTUS is available as a cell suspension for infusion. MCL: A single dose of TECARTUS contains 2 × 106 CAR-positive viable T cells per kg of body weight [maximum of 2 × 108 CAR-positive viable ...
-
4 CONTRAINDICATIONSNone.
-
5 WARNINGS AND PRECAUTIONS5.1 Cytokine Release Syndrome - CRS, including fatal or life-threatening reactions, occurred following treatment with TECARTUS. CRS occurred in 91% (75/82) of patients with MCL, including ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Cytokine Release Syndrome [see Warnings and Precautions (5.1)] Neurologic Toxicities [see Warnings ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available data with TECARTUS use in pregnant women. No animal reproductive and developmental toxicity studies have been conducted with TECARTUS to ...
-
11 DESCRIPTIONTECARTUS (brexucabtagene autoleucel) is a CD19-directed genetically modified autologous T cell immunotherapy. To prepare TECARTUS, a patient's own T cells are harvested and genetically modified ex ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - TECARTUS, a CD19-directed genetically modified autologous T cell immunotherapy, binds to CD19-expressing cancer cells and normal B cells. Studies demonstrated that ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No carcinogenicity or genotoxicity studies have been conducted with TECARTUS. No studies have been conducted to evaluate the effects of ...
-
14 CLINICAL STUDIES14.1 Relapsed or Refractory Mantle Cell Lymphoma - A single-arm, open-label, multicenter trial (ZUMA-2; NCT02601313) evaluated the efficacy and safety of a single infusion of TECARTUS in adult ...
-
15 REFERENCESLee DW et al (2014). Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014 Jul 10; 124(2): 188–195.
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTECARTUS is supplied in an infusion bag containing approximately 68 mL of frozen suspension of genetically modified autologous T cells in 5% DMSO and human serum albumin. Each TECARTUS infusion ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Ensure that patients understand the risk of manufacturing failure (4% in clinical trial). In case of a ...
-
SPL UNCLASSIFIED SECTIONManufactured by, Packed by, Distributed by: Kite Pharma, Inc. Santa Monica, CA 90404 - US License No 2064 - © 2024 Kite Pharma, Inc. All Rights Reserved. 125703-GS-005
-
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: June 2024 - MEDICATION GUIDE - TECARTUS (pronounced tek-ahr-tuhs) (brexucabtagene autoleucel) Read ...
-
PRINCIPAL DISPLAY PANEL - 68 mL Bag Label - Product - AS-02685NDC 71287-219-01 - brexucabtagene autoleucel - TECARTUS® Rx ONLY - FOR AUTOLOGOUS & INTRAVENOUS USE ONLY - No U.S. standard of potency - Dose: One sterile bag for infusion. Contents: Maximum of 2 x 108 ...
-
PRINCIPAL DISPLAY PANEL - 68 mL Cassette Label - Product - AS-02860NDC 71287-219-02 - brexucabtagene autoleucel - TECARTUS® Rx ONLY - FOR AUTOLOGOUS & INTRAVENOUS USE ONLY - No U.S. standard of potency - Dose: One sterile bag for infusion. Contents: Maximum of 2 x 108 ...
-
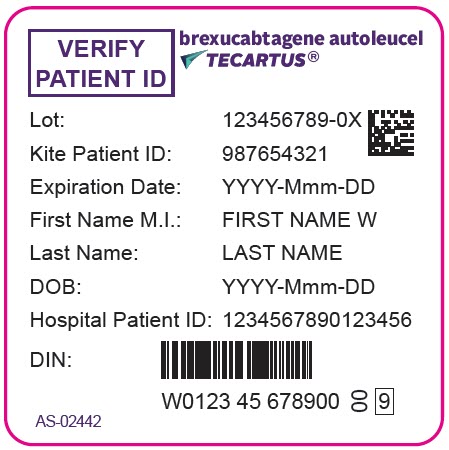
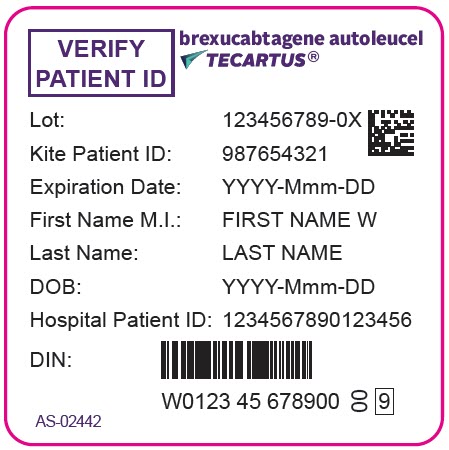
PRINCIPAL DISPLAY PANEL - 68 mL Bag Label - Patient - AS-02442VERIFY - PATIENT ID - brexucabtagene autoleucel - TECARTUS® Lot: 123456789-0X - Kite Patient ID: 987654321 - Expiration Date: YYYY-Mmm-DD - First Name M.I.: FIRST NAME W - Last Name: LAST NAME - DOB ...
-
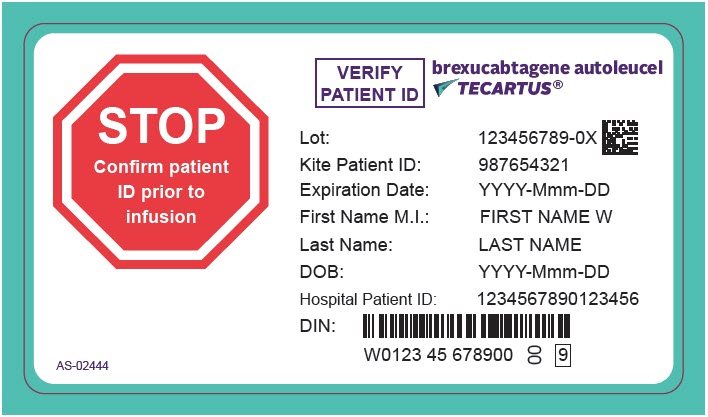
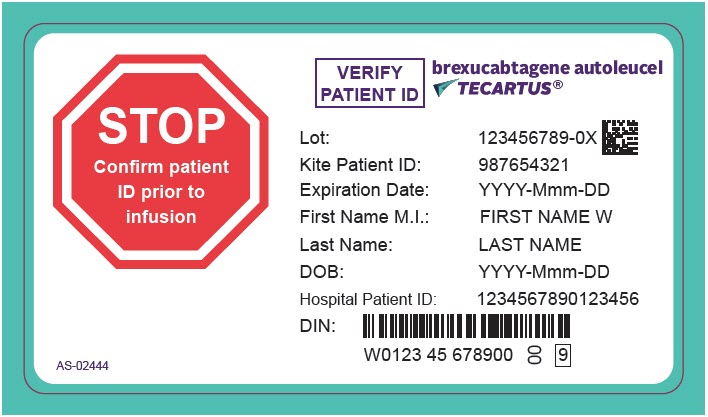
PRINCIPAL DISPLAY PANEL - 68 mL Cassette Label - Patient - AS-02444STOP - Confirm patient - ID prior to - infusion - VERIFY - PATIENT ID - brexucabtagene autoleucel - TECARTUS® Lot: 123456789-0X - Kite Patient ID: 987654321 - Expiration Date: YYYY-Mmm-DD - First Name M.I.: FIRST ...
-
PRINCIPAL DISPLAY PANEL - 68 mL LN2 Label - AS-02447brexucabtagene autoleucel - TECARTUS® Kite Pharma, Inc. Site: FXX - Expiration Date: YYYY-Mmm-DD - Cell Order: 123456789 - LOT: 123456789-01 - Ship and store in vapor phase of liquid nitrogen ≤ ...
-
PRINCIPAL DISPLAY PANEL - 68 mL Bag Label - Product - AS-02535NDC 71287-220-01 - brexucabtagene autoleucel - TECARTUS® Rx ONLY - FOR AUTOLOGOUS & INTRAVENOUS USE ONLY - No U.S. standard of potency - Dose: One sterile bag for infusion. Contents: Maximum of 1 x 108 ...
-
PRINCIPAL DISPLAY PANEL - 68 mL Cassette Label - Product - AS-02861NDC 71287-220-02 - brexucabtagene autoleucel - TECARTUS® Rx ONLY - FOR AUTOLOGOUS & INTRAVENOUS USE ONLY - No U.S. standard of potency - Dose: One sterile bag for infusion. Contents: Maximum of 1 x 108 ...
-
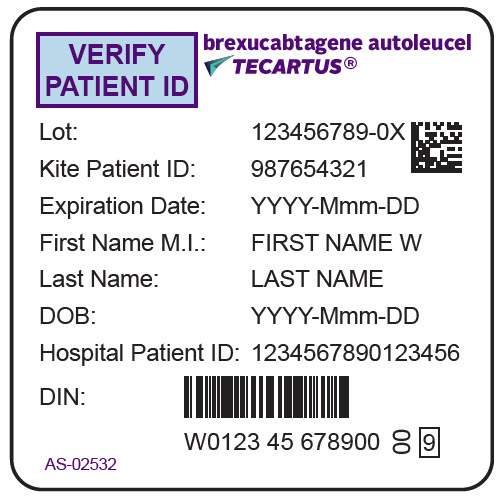
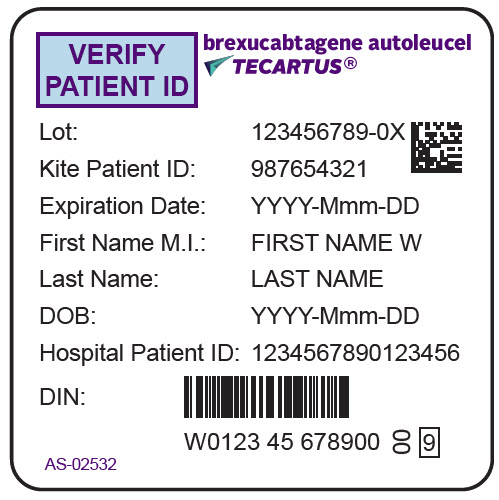
PRINCIPAL DISPLAY PANEL - 68 mL Bag Label - Patient - AS-02532VERIFY - PATIENT ID - brexucabtagene autoleucel - TECARTUS® Lot: 123456789-0X - Kite Patient ID: 987654321 - Expiration Date: YYYY-Mmm-DD - First Name M.I.: FIRST NAME W - Last Name: LAST NAME - DOB ...
-
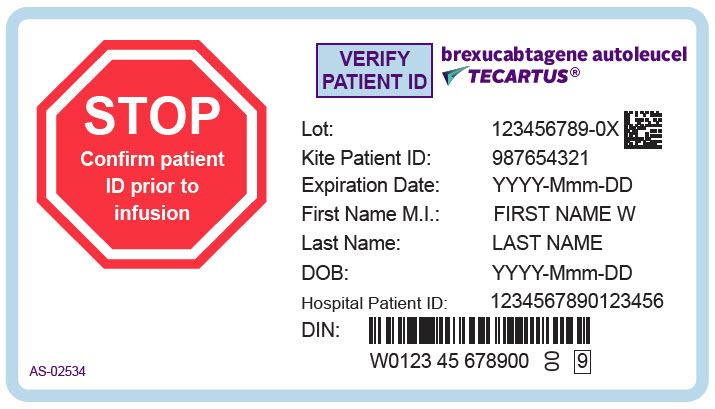
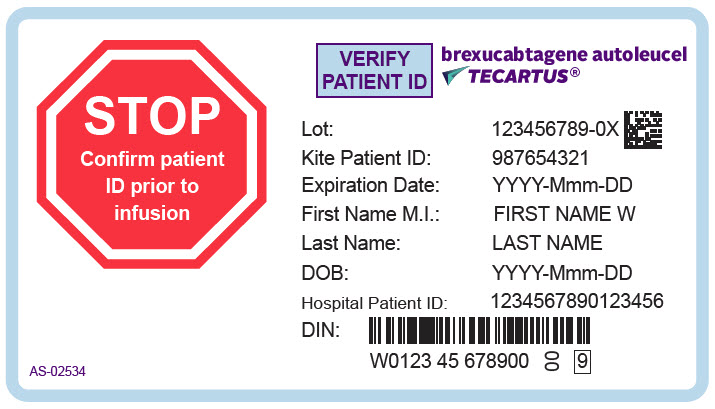
PRINCIPAL DISPLAY PANEL - 68 mL Cassette Label - Patient - AS-02534STOP - Confirm patient - ID prior to - infusion - VERIFY - PATIENT ID - brexucabtagene autoleucel - TECARTUS® Lot: 123456789-0X - Kite Patient ID: 987654321 - Expiration Date: YYYY-Mmm-DD - First Name M.I.: FIRST ...
-
PRINCIPAL DISPLAY PANEL - 68 mL LN2 Label - AS-02536brexucabtagene autoleucel - TECARTUS® Kite Pharma, Inc. Site: FXX - Expiration Date: YYYY-Mmm-DD - Cell Order: 123456789 - LOT: 123456789-01 - Ship and store in vapor phase of liquid nitrogen ≤ -150°C - 2400 ...
-
INGREDIENTS AND APPEARANCEProduct Information