Label: BLINCYTO- blinatumomab kit
- NDC Code(s): 55513-150-01, 55513-155-01, 55513-160-01
- Packager: Amgen Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated December 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BLINCYTO® safely and effectively. See full prescribing information for BLINCYTO. BLINCYTO® (blinatumomab) for injection, for ...These highlights do not include all the information needed to use BLINCYTO® safely and effectively. See full prescribing information for BLINCYTO.
BLINCYTO® (blinatumomab) for injection, for intravenous use
Initial U.S. Approval: 2014WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGICAL TOXICITIES including IMMUNE EFFECTOR CELL-ASSOCIATED NEUROTOXICITY SYNDROME
See full prescribing information for complete boxed warning.
- Cytokine Release Syndrome (CRS), which may be life-threatening or fatal, occurred in patients receiving BLINCYTO. Interrupt or discontinue BLINCYTO and treat with corticosteroids as recommended. (2.4, 5.1)
- Neurological toxicities, including immune effector cell-associated neurotoxicity syndrome (ICANS), which may be severe, life-threatening, or fatal, occurred in patients receiving BLINCYTO. Interrupt or discontinue BLINCYTO as recommended. (2.4, 5.2)
RECENT MAJOR CHANGES
Boxed Warning 2/2024 Indications and Usage (1.1, 1.2, 1.3) 6/2024 Dosage and Administration (2.1, 2.2, 2.3) 12/2024 Dosage and Administration (2.4, 2.5, 2.6, 2.7, 2.8) 12/2024 Warnings and Precautions, Cytokine Release Syndrome (5.1) 6/2024 Warnings and Precautions, Neurological Toxicities including Immune Effector Cell-Associated Neurotoxicity (5.2) 6/2024 Warnings and Precautions, Effects on Ability to Drive and Use Machines (5.6) 2/2024 Warnings and Precautions, Benzyl Alcohol Toxicity in Neonates (5.12) 12/2024 INDICATIONS AND USAGE
BLINCYTO is a bispecific CD19-directed CD3 T-cell engager indicated for the treatment of adult and pediatric patients one month and older with:
- CD19-positive B-cell precursor acute lymphoblastic leukemia (ALL) in first or second complete remission with minimal residual disease (MRD) greater than or equal to 0.1%. (1.1)
- Relapsed or refractory CD19-positive B-cell precursor acute lymphoblastic leukemia (ALL). (1.2)
- CD19-positive Philadelphia chromosome-negative B-cell precursor acute lymphoblastic leukemia (ALL) in the consolidation phase of multiphase chemotherapy. (1.3)
DOSAGE AND ADMINISTRATION
- For the treatment of MRD-positive B-cell Precursor ALL
- For the treatment of Relapsed or Refractory B-cell Precursor ALL
- For the treatment of B-cell Precursor ALL in the Consolidation Phase
- Refer to Full Prescribing Information for important preparation and administration information. (2.5)
- Administer as a continuous intravenous infusion at a constant flow rate using an infusion pump.
- -
- See Instructions for Use for infusion over 24 hours or 48 hours.
- -
- See Instructions for Use for infusion over 72 hours, 96 hours, or 7 days using Bacteriostatic 0.9% Sodium Chloride Injection (containing 0.9% benzyl alcohol). This option is not recommended for patients weighing less than 5.4 kg.
DOSAGE FORMS AND STRENGTHS
For injection: 35 mcg of lyophilized powder in a single-dose vial for reconstitution. (3)
CONTRAINDICATIONS
Known hypersensitivity to blinatumomab or to any component of the product formulation. (4)
WARNINGS AND PRECAUTIONS
- Infections: Monitor patients for signs or symptoms; treat appropriately. (5.3)
- Effects on Ability to Drive and Use Machines: Advise patients to refrain from driving and engaging in hazardous occupations or activities such as operating heavy or potentially dangerous machinery while BLINCYTO is being administered. (5.6)
- Pancreatitis: Evaluate patients who develop signs and symptoms of pancreatitis. Management of pancreatitis may require either temporary interruption or discontinuation of BLINCYTO. (5.8)
- Preparation and Administration Errors: Strictly follow instructions for preparation (including admixing) and administration. (5.10)
- Benzyl Alcohol Toxicity in Neonates: Use BLINCYTO prepared with preservative-free saline for neonates. BLINCYTO solution containing benzyl alcohol is not recommended for patients weighing less than 5.4 kg. (5.12, 8.4)
- Embryo-Fetal Toxicity: May cause fetal harm. Advise females of reproductive potential of the potential risk to the fetus and to use effective contraception. (5.13, 8.1, 8.3)
ADVERSE REACTIONS
The most common adverse reactions (≥ 20%) are pyrexia, infusion-related reactions, headache, infection, musculoskeletal pain, neutropenia, nausea, anemia, thrombocytopenia, and diarrhea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Amgen Inc. at 1-800-77-AMGEN (1-800-772-6436) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 12/2024
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGICAL TOXICITIES including IMMUNE EFFECTOR CELL-ASSOCIATED NEUROTOXICITY SYNDROME
1 INDICATIONS AND USAGE
1.1 MRD-positive B-cell Precursor ALL
1.2 Relapsed or Refractory B-cell Precursor ALL
1. 3 B-cell Precursor ALL in the Consolidation Phase
2 DOSAGE AND ADMINISTRATION
2.1 Treatment of MRD-positive B-cell Precursor ALL
2.2 Treatment of Relapsed or Refractory B-cell Precursor ALL
2.3 Treatment of B-cell Precursor ALL in the Consolidation Phase
2.4 Dosage Modifications for Adverse Reactions
2.5 Preparation and Administration of BLINCYTO
2.6 Storage of Reconstituted BLINCYTO
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Cytokine Release Syndrome
5.2 Neurological Toxicities, including Immune Effector Cell-Associated Neurotoxicity Syndrome
5.3 Infections
5.4 Tumor Lysis Syndrome
5.5 Neutropenia and Febrile Neutropenia
5.6 Effects on Ability to Drive and Use Machines
5.7 Elevated Liver Enzymes
5.8 Pancreatitis
5.9 Leukoencephalopathy
5.10 Preparation and Administration Errors
5.11 Immunization
5.12 Benzyl Alcohol Toxicity in Neonates
5.13 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.6 Immunogenicity
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 MRD-positive B-cell Precursor ALL
14.2 Relapsed/Refractory B-cell Precursor ALL
14.3 Philadelphia Chromosome-Negative B-cell Precursor ALL in the Consolidation Phase
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGICAL TOXICITIES including IMMUNE EFFECTOR CELL-ASSOCIATED NEUROTOXICITY SYNDROME
- Cytokine Release Syndrome (CRS), which may be life-threatening or fatal, occurred in patients receiving BLINCYTO. Interrupt or discontinue BLINCYTO and treat with corticosteroids as recommended [see Dosage and Administration (2.4), Warnings and Precautions (5.1)].
- Neurological toxicities, including immune effector cell-associated neurotoxicity syndrome (ICANS) which may be severe, life-threatening, or fatal, occurred in patients receiving BLINCYTO. Interrupt or discontinue BLINCYTO as recommended. [see Dosage and Administration (2.4), Warnings and Precautions (5.2)].
-
1 INDICATIONS AND USAGE1.1 MRD-positive B-cell Precursor ALL - BLINCYTO is indicated for the treatment of CD19-positive B-cell precursor acute lymphoblastic leukemia (ALL) in first or second complete remission with ...
1.1 MRD-positive B-cell Precursor ALL
BLINCYTO is indicated for the treatment of CD19-positive B-cell precursor acute lymphoblastic leukemia (ALL) in first or second complete remission with minimal residual disease (MRD) greater than or equal to 0.1% in adult and pediatric patients one month and older.
1.2 Relapsed or Refractory B-cell Precursor ALL
BLINCYTO is indicated for the treatment of relapsed or refractory CD19-positive B-cell precursor acute lymphoblastic leukemia (ALL) in adult and pediatric patients one month and older.
Close1. 3 B-cell Precursor ALL in the Consolidation Phase
BLINCYTO is indicated for the treatment of CD19-positive Philadelphia chromosome-negative B-cell precursor acute lymphoblastic leukemia (ALL) in the consolidation phase of multiphase chemotherapy in adult and pediatric patients one month and older.
-
2 DOSAGE AND ADMINISTRATION2.1 Treatment of MRD-positive B-cell Precursor ALL - A treatment course consists of 1 cycle of BLINCYTO for induction followed by up to 3 additional cycles for consolidation. A single cycle of ...
2.1 Treatment of MRD-positive B-cell Precursor ALL
- A treatment course consists of 1 cycle of BLINCYTO for induction followed by up to 3 additional cycles for consolidation.
- A single cycle of treatment of BLINCYTO induction or consolidation consists of 28 days of continuous intravenous infusion followed by a 14-day treatment-free interval (total 42 days).
- See Table 1 for the recommended dose by patient weight and schedule. Patients weighing 45 kg or more receive a fixed-dose. For patients weighing less than 45 kg, the dose is calculated using the patient's body surface area (BSA).
Table 1. Recommended BLINCYTO Dose and Schedule for the Treatment of MRD-positive B-cell Precursor ALL Cycle Patients Weighing 45 kg or More
(Fixed-dose)Patients Weighing Less Than 45 kg
(BSA-based dose)Induction Cycle 1 Days 1-28 28 mcg/day 15 mcg/m2/day
(not to exceed 28 mcg/day)
Days 29-42 14-day treatment-free interval
14-day treatment-free interval Consolidation Cycles 2-4
Days 1-28
28 mcg/day 15 mcg/m2/day
(not to exceed 28 mcg/day)
Days 29-42 14-day treatment-free interval
14-day treatment-free interval - Hospitalization is recommended for the first 3 days of the first cycle and the first 2 days of the second cycle. For all subsequent cycle starts and re-initiations (e.g., if treatment is interrupted for 4 or more hours), supervision by a healthcare professional or hospitalization is recommended.
- Intrathecal chemotherapy prophylaxis is recommended before and during BLINCYTO therapy to prevent central nervous system ALL relapse.
-
Premedicate with prednisone or equivalent for MRD-positive B-cell Precursor ALL:
- For adult patients, premedicate with prednisone 100 mg intravenously or equivalent (e.g., dexamethasone 16 mg) 1 hour prior to the first dose of BLINCYTO in each cycle.
- For pediatric patients, premedicate with 5 mg/m2 of dexamethasone intravenously or orally, to a maximum dose of 20 mg, prior to the first dose of BLINCYTO in the first cycle and when restarting an infusion after an interruption of 4 or more hours in the first cycle.
-
For administration of BLINCYTO:
- See Instructions for Use for infusion over 24 hours or 48 hours.
- See Instructions for Use for infusion over 72 hours, 96 hours, or 7 days using Bacteriostatic 0.9% Sodium Chloride Injection (containing 0.9% benzyl alcohol). The administration of BLINCYTO as a 72-hour, 96-hour, and 7-day infusion is not recommended for patients weighing less than 5.4 kg.
2.2 Treatment of Relapsed or Refractory B-cell Precursor ALL
- A treatment course consists of up to 2 cycles of BLINCYTO for induction followed by 3 additional cycles for consolidation and up to 4 additional cycles of continued therapy.
- A single cycle of treatment of BLINCYTO induction or consolidation consists of 28 days of continuous intravenous infusion followed by a 14-day treatment-free interval (total 42 days).
- A single cycle of treatment of BLINCYTO continued therapy consists of 28 days of continuous intravenous infusion followed by a 56-day treatment-free interval (total 84 days).
- See Table 2 for the recommended dose by patient weight and schedule. Patients weighing 45 kg or more receive a fixed-dose and for patients weighing less than 45 kg, the dose is calculated using the patient's BSA.
Table 2. Recommended BLINCYTO Dose and Schedule for the Treatment of Relapsed or Refractory B-cell Precursor ALL Cycle Patients Weighing 45 kg or More
(Fixed-dose)Patients Weighing Less Than 45 kg
(BSA-based dose)Induction Cycle 1 Days 1-7 9 mcg/day 5 mcg/m2/day
(not to exceed 9 mcg/day)
Days 8-28 28 mcg/day 15 mcg/m2/day
(not to exceed 28 mcg/day)
Days 29-42 14-day treatment-free interval 14-day treatment-free interval
Induction Cycle 2
Days 1-28 28 mcg/day 15 mcg/m2/day
(not to exceed 28 mcg/day)
Days 29-42 14-day treatment-free interval 14-day treatment-free interval
Consolidation Cycles 3-5
Days 1-28 28 mcg/day 15 mcg/m2/day
(not to exceed 28 mcg/day)
Days 29-42 14-day treatment-free interval 14-day treatment-free interval
Continued Therapy Cycles 6-9 Days 1-28 28 mcg/day 15 mcg/m2/day
(not to exceed 28 mcg/day)
Days 29-84 56-day treatment-free interval 56-day treatment-free interval
- Hospitalization is recommended for the first 9 days of the first cycle and the first 2 days of the second cycle. For all subsequent cycle starts and re-initiations (e.g., if treatment is interrupted for 4 or more hours), supervision by a healthcare professional or hospitalization is recommended.
- Intrathecal chemotherapy prophylaxis is recommended before and during BLINCYTO therapy to prevent central nervous system ALL relapse.
-
Premedicate with dexamethasone:
- For adult patients, premedicate with 20 mg of dexamethasone intravenously or orally 1 hour prior to the first dose of BLINCYTO of each cycle, prior to a step dose (such as Cycle 1 Day 8), and when restarting an infusion after an interruption of 4 or more hours.
- For pediatric patients, premedicate with 5 mg/m2 of dexamethasone intravenously or orally, to a maximum dose of 20 mg, prior to the first dose of BLINCYTO in the first cycle, prior to a step dose (such as Cycle 1 Day 8), and when restarting an infusion after an interruption of 4 or more hours in the first cycle.
- For administration of BLINCYTO:
- See Instructions for Use for infusion over 24 hours or 48 hours.
- See Instructions for Use for infusion over 72 hours, 96 hours, or 7 days using Bacteriostatic 0.9% Sodium Chloride Injection (containing 0.9% benzyl alcohol). The administration of BLINCYTO as a 72-hour, 96-hour, and 7-day infusion is not recommended for patients weighing less than 5.4 kg.
2.3 Treatment of B-cell Precursor ALL in the Consolidation Phase
- A single cycle of BLINCYTO monotherapy in consolidation is 28 days of continuous infusion followed by a 14-day treatment-free interval (total 42 days) [see Table 3 and Clinical Studies (14.3)].
- Patients weighing 45 kg or more receive a fixed-dose, and for patients weighing less than 45 kg, the dose is calculated using the patient's BSA (see Table 3).
Table 3. Recommended BLINCYTO Dose and Schedule in the Consolidation Phase of Treatment of B-cell Precursor ALL BLINCYTO Consolidation Cycle Patients Weighing 45 kg or More
(Fixed-dose)Patients Weighing Less Than 45 kg
(BSA-based dose)Days 1-28 28 mcg/day 15 mcg/m2/day
(not to exceed 28 mcg/day)Days 29-42 14-day treatment-free interval 14-day treatment-free interval - Hospitalization is recommended for the first 3 days of the first cycle and the first 2 days of the second cycle. For all subsequent cycle starts and re-initiations (e.g., if treatment is interrupted for 4 or more hours), supervision by a healthcare professional or hospitalization is recommended.
- Intrathecal chemotherapy prophylaxis is recommended before and during BLINCYTO therapy to prevent central nervous system ALL relapse.
-
Premedicate with dexamethasone:
- For adult patients, premedicate with dexamethasone 20 mg intravenously within 1 hour prior to the first dose of BLINCYTO of each cycle.
- For pediatric patients, premedicate with 5 mg/m2 of dexamethasone intravenously or orally, to a maximum dose of 20 mg prior to the first dose of BLINCYTO in the first cycle and when restarting an infusion after an interruption of 4 or more hours in the first cycle.
-
For administration of BLINCYTO:
- See Instructions for Use for infusion over 24 hours or 48 hours.
- See Instructions for Use for infusion over 72 hours, 96 hours, or 7 days using Bacteriostatic 0.9% Sodium Chloride Injection (containing 0.9% benzyl alcohol). The administration of BLINCYTO as a 72-hour, 96-hour, and 7-day infusion is not recommended for patients weighing less than 5.4 kg.
2.4 Dosage Modifications for Adverse Reactions
If the interruption after an adverse reaction is no longer than 7 days, continue the same cycle to a total of 28 days of infusion inclusive of days before and after the interruption in that cycle. If an interruption due to an adverse reaction is longer than 7 days, start a new cycle.
Table 4. Dosage Modifications for Adverse Reactions Adverse Reaction Grade* Patients Weighing 45 kg or More Patients Weighing Less Than 45 kg - *
- Based on the Common Terminology Criteria for Adverse Events (CTCAE). Grade 3 is severe, and Grade 4 is life-threatening.
Cytokine Release Syndrome (CRS) Grade 3 - Interrupt BLINCYTO.
- Administer dexamethasone 8 mg every 8 hours intravenously or orally for up to 3 days and taper thereafter over 4 days.
- When CRS is resolved, restart BLINCYTO at 9 mcg/day, and escalate to 28 mcg/day after 7 days if the adverse reaction does not recur.
- Interrupt BLINCYTO.
- Administer dexamethasone 5 mg/m2 (maximum 8 mg) every 8 hours intravenously or orally for up to 3 days and taper thereafter over 4 days.
- When CRS is resolved, restart BLINCYTO at 5 mcg/m2/day, and escalate to 15 mcg/m2/day after 7 days if the adverse reaction does not recur.
Grade 4 Discontinue BLINCYTO permanently. Administer dexamethasone as instructed for Grade 3 CRS. Neurological Toxicity Seizure Discontinue BLINCYTO permanently if more than one seizure occurs. Grade 2 ICANS Interrupt BLINCYTO until ICANS resolves.
Administer corticosteroids and manage according to current practice guidelines.
When ICANS is resolved, restart BLINCYTO at 9 mcg/day.
Escalate to 28 mcg/day after 7 days if the adverse reaction does not recur.Interrupt BLINCYTO until ICANS resolves.
Administer corticosteroids and manage according to current practice guidelines.
When ICANS is resolved, restart BLINCYTO at 5 mcg/m2/day.
Escalate to 15 mcg/m2/day after 7 days if the adverse reaction does not recur.Grade 3 Neurologic Events including ICANS Withhold BLINCYTO until no more than Grade 1 (mild) and for at least 3 days, then restart BLINCYTO at 9 mcg/day. Escalate to 28 mcg/day after 7 days if the adverse reaction does not recur. If the adverse reaction occurred at 9 mcg/day, or if the adverse reaction takes more than 7 days to resolve, discontinue BLINCYTO permanently. Withhold BLINCYTO until no more than Grade 1 (mild) and for at least 3 days, then restart BLINCYTO at 5 mcg/m2/day. Escalate to 15 mcg/m2/day after 7 days if the adverse reaction does not recur. If the adverse reaction occurred at 5 mcg/m2/day, or if the adverse reaction takes more than 7 days to resolve, discontinue BLINCYTO permanently. If ICANS, administer corticosteroids and manage according to current practice guidelines. Grade 4 Discontinue BLINCYTO permanently. Neurologic Events including ICANS If ICANS, administer corticosteroids and manage according to current practice guidelines. Other Clinically Relevant Adverse Reactions Grade 3 Withhold BLINCYTO until no more than Grade 1 (mild), then restart BLINCYTO at 9 mcg/day. Escalate to 28 mcg/day after 7 days if the adverse reaction does not recur. If the adverse reaction takes more than 14 days to resolve, discontinue BLINCYTO permanently. Withhold BLINCYTO until no more than Grade 1 (mild), then restart BLINCYTO at 5 mcg/m2/day. Escalate to 15 mcg/m2/day after 7 days if the adverse reaction does not recur. If the adverse reaction takes more than 14 days to resolve, discontinue BLINCYTO permanently. Grade 4 Consider discontinuing BLINCYTO permanently. 2.5 Preparation and Administration of BLINCYTO
It is very important that the instructions for preparation (including admixing) and administration provided in this section are strictly followed to minimize medication errors (including underdose and overdose) [see Warnings and Precautions (5.10)].
BLINCYTO can be infused over 24 hours (preservative-free), 48 hours (preservative-free), 72 hours (with preservative), 96 hours (with preservative), or 7 days (with preservative). The choice between these options for the infusion duration should be made by the treating healthcare provider considering the frequency of the infusion bag changes and the weight of the patient. The administration of BLINCYTO as a 72-hour, 96-hour, and 7-day infusion is not recommended for patients weighing less than 5.4 kg.
For preparation, reconstitution, and administration of BLINCYTO:
The BLINCYTO Instructions for Use contains more detailed instructions on the preparation of infusion [see Instructions for Use].
The preparation steps differ based on the infusion duration. Follow the steps specific to the infusion duration you are preparing.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Call 1-800-77-AMGEN (1-800-772-6436) if you have questions about the reconstitution and preparation of BLINCYTO.
Close2.6 Storage of Reconstituted BLINCYTO
The information in Table 5 indicates the storage time for the reconstituted BLINCYTO vial and prepared infusion bag.
Table 5. Storage Time for Reconstituted BLINCYTO Vial and Prepared BLINCYTO Infusion Bag Maximum Storage Time Room Temperature
23°C to 27°C
(73°F to 81°F)Refrigerated
2°C to 8°C
(36°F to 46°F)- *
- Storage time includes infusion time. If the prepared BLINCYTO infusion bag is not administered within the time frames and temperatures indicated, it must be discarded; it should not be refrigerated again.
Reconstituted BLINCYTO Vial 4 hours 24 hours Prepared BLINCYTO 24-Hour and 48-Hour Infusion Bag
(Preservative-free)48 hours* 8 days Prepared BLINCYTO 72-Hour and 96-Hour Infusion Bag
(with Preservative)4 days* 14 days Prepared BLINCYTO 7-Day Infusion Bag
(with Preservative)7 days* 14 days -
3 DOSAGE FORMS AND STRENGTHSFor injection: 35 mcg of white to off-white lyophilized powder in a single-dose vial for reconstitution.
For injection: 35 mcg of white to off-white lyophilized powder in a single-dose vial for reconstitution.
Close -
4 CONTRAINDICATIONSBLINCYTO is contraindicated in patients with known hypersensitivity to blinatumomab or to any component of the product formulation.
BLINCYTO is contraindicated in patients with known hypersensitivity to blinatumomab or to any component of the product formulation.
Close -
5 WARNINGS AND PRECAUTIONS5.1 Cytokine Release Syndrome - Cytokine Release Syndrome (CRS), which may be life-threatening or fatal, occurred in patients receiving BLINCYTO. The median time to onset of CRS was 2 days after ...
5.1 Cytokine Release Syndrome
Cytokine Release Syndrome (CRS), which may be life-threatening or fatal, occurred in patients receiving BLINCYTO. The median time to onset of CRS was 2 days after the start of infusion and the median time to resolution of CRS was 5 days among cases that resolved. Manifestations of CRS include fever, headache, nausea, asthenia, hypotension, increased alanine aminotransferase (ALT), increased aspartate aminotransferase (AST), increased total bilirubin, and disseminated intravascular coagulation (DIC). The manifestations of CRS after treatment with BLINCYTO overlap with those of infusion reactions, capillary leak syndrome (CLS), and hemophagocytic histiocytosis/macrophage activation syndrome (MAS). Using all of these terms to define CRS in clinical trials of BLINCYTO, CRS was reported in 15% of patients with relapsed or refractory ALL, in 7% of patients with MRD-positive ALL, and in 16% of patients receiving BLINCYTO cycles in the consolidation phase of therapy [see Adverse Reactions (6.1)].
Monitor patients for signs or symptoms of these events. Advise outpatients on BLINCYTO to contact their healthcare professional for signs and symptoms associated with CRS. If severe CRS occurs, interrupt BLINCYTO until CRS resolves. Discontinue BLINCYTO permanently if life-threatening CRS occurs. Administer corticosteroids for severe or life-threatening CRS [see Dosage and Administration (2.4)].
5.2 Neurological Toxicities, including Immune Effector Cell-Associated Neurotoxicity Syndrome
BLINCYTO can cause serious or life-threatening neurologic toxicity, including ICANS [see Adverse Reactions 6.1].
The incidence of neurologic toxicities in clinical trials was approximately 65% [see Adverse Reactions (6.1)]. Among patients that experienced a neurologic toxicity, the median time to the first event was within the first 2 weeks of BLINCYTO treatment. The most common (≥ 10%) manifestations of neurological toxicity were headache, and tremor; the neurological toxicity profile varied by age group [see Use in Specific Populations (8.4, 8.5)]. Grade 3 or higher neurological toxicities following initiation of BLINCYTO administration occurred in approximately 13% of patients and included encephalopathy, convulsions, speech disorders, disturbances in consciousness, confusion and disorientation, and coordination and balance disorders. Manifestations of neurological toxicity included cranial nerve disorders. The majority of neurologic toxicities resolved following interruption of BLINCYTO, but some resulted in treatment discontinuation.
The incidence of signs and symptoms consistent with ICANS in clinical trials was 7.5%. The onset of ICANS can be concurrent with CRS, following resolution of CRS, or in the absence of CRS.
There is limited experience with BLINCYTO in patients with active ALL in the central nervous system (CNS) or a history of neurologic events. Patients with a history or presence of clinically relevant CNS pathology were excluded from clinical studies. Patients with Down Syndrome may have a higher risk of seizures with BLINCYTO therapy.
Monitor patients receiving BLINCYTO for signs and symptoms of neurological toxicities, including ICANS. Advise outpatients on BLINCYTO to contact their healthcare professional if they develop signs or symptoms of neurological toxicities. Management of neurologic toxicity may require interruption or discontinuation of BLINCYTO as recommended and/or treatment with corticosteroids [see Dosage and Administration (2.4)].
5.3 Infections
In patients with ALL receiving BLINCYTO in clinical studies, serious infections such as sepsis, pneumonia, bacteremia, opportunistic infections, and catheter-site infections were observed in approximately 25% of patients, some of which were life-threatening or fatal [see Adverse Reactions (6.1)]. As appropriate, administer prophylactic antibiotics and employ surveillance testing during treatment with BLINCYTO. Monitor patients for signs and symptoms of infection and treat appropriately.
5.4 Tumor Lysis Syndrome
Tumor lysis syndrome (TLS), which may be life-threatening or fatal, has been observed in patients receiving BLINCYTO [see Adverse Reactions (6.1)]. Appropriate prophylactic measures, including pretreatment nontoxic cytoreduction and on-treatment hydration, should be used for the prevention of TLS during BLINCYTO treatment. Monitor for signs or symptoms of TLS. Management of these events may require either temporary interruption or discontinuation of BLINCYTO [see Dosage and Administration (2.4)].
5.5 Neutropenia and Febrile Neutropenia
Neutropenia and febrile neutropenia, including life-threatening cases, have been observed in patients receiving BLINCYTO [see Adverse Reactions (6.1)]. Monitor laboratory parameters (including, but not limited to, white blood cell count and absolute neutrophil count) during BLINCYTO infusion. Interrupt BLINCYTO if prolonged neutropenia occurs.
5.6 Effects on Ability to Drive and Use Machines
Due to the potential for neurologic events, including seizures and ICANS, patients receiving BLINCYTO are at risk for loss of consciousness [see Warnings and Precautions (5.2)]. Advise patients to refrain from driving and engaging in hazardous occupations or activities such as operating heavy or potentially dangerous machinery while BLINCYTO is being administered.
5.7 Elevated Liver Enzymes
Treatment with BLINCYTO was associated with transient elevations in liver enzymes [see Adverse Reactions (6.1)]. In patients with ALL receiving BLINCYTO in clinical studies, the median time to onset of elevated liver enzymes was 3 days.
The majority of these transient elevations in liver enzymes were observed in the setting of CRS. For the events that were observed outside the setting of CRS, the median time to onset was 19 days. Grade 3 or greater elevations in liver enzymes occurred in approximately 7% of patients outside the setting of CRS and resulted in treatment discontinuation in less than 1% of patients.
Monitor alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), and total blood bilirubin prior to the start of and during BLINCYTO treatment. Interrupt BLINCYTO if the transaminases rise to greater than 5 times the upper limit of normal or if total bilirubin rises to more than 3 times the upper limit of normal.
5.8 Pancreatitis
Fatal pancreatitis has been reported in patients receiving BLINCYTO in combination with dexamethasone in clinical studies and the postmarketing setting [see Adverse Reactions (6.2)].
Evaluate patients who develop signs and symptoms of pancreatitis. Management of pancreatitis may require either temporary interruption or discontinuation of BLINCYTO and dexamethasone [see Dosage and Administration (2.4)].
5.9 Leukoencephalopathy
Cranial magnetic resonance imaging (MRI) changes showing leukoencephalopathy have been observed in patients receiving BLINCYTO, especially in patients with prior treatment with cranial irradiation and antileukemic chemotherapy (including systemic high-dose methotrexate or intrathecal cytarabine). The clinical significance of these imaging changes is unknown.
5.10 Preparation and Administration Errors
Preparation and administration errors have occurred with BLINCYTO treatment. Follow instructions for preparation (including admixing) and administration strictly to minimize medication errors (including underdose and overdose) [see Dosage and Administration (2.5) and Instructions for Use].
5.11 Immunization
The safety of immunization with live viral vaccines during or following BLINCYTO therapy has not been studied. Vaccination with live virus vaccines is not recommended for at least 2 weeks prior to the start of BLINCYTO treatment, during treatment, and until immune recovery following last cycle of BLINCYTO.
5.12 Benzyl Alcohol Toxicity in Neonates
Serious adverse reactions, including fatal reactions and the "gasping syndrome," have been reported in very low birth weight (VLBW) neonates born weighing less than 1500 g, and early preterm neonates (infants born less than 34 weeks gestational age) who received intravenous drugs containing benzyl alcohol as a preservative. Early preterm VLBW neonates may be more likely to develop these reactions, because they may be less able to metabolize benzyl alcohol [see Use in Specific Populations (8.4)].
Use the preservative-free preparations of BLINCYTO where possible in neonates. When prescribing BLINCYTO (with preservative) for neonatal patients, consider the combined daily metabolic load of benzyl alcohol from all sources including BLINCYTO (with preservative), other products containing benzyl alcohol or other excipients (e.g., ethanol, propylene glycol) which compete with benzyl alcohol for the same metabolic pathway.
Monitor neonatal patients receiving BLINCYTO (with preservative) for new or worsening metabolic acidosis. The minimum amount of benzyl alcohol at which serious adverse reactions may occur in neonates is not known. The BLINCYTO 72-Hour bag (with preservative) and 96-Hour bag (with preservative) contain 2.5 mg of benzyl alcohol per mL, and the 7-Day bag (with preservative) contains 7.4 mg of benzyl alcohol per mL. The administration of BLINCYTO as a 72-hour, 96-hour, and 7-day infusion is not recommended for patients weighing less than 5.4 kg [see Use in Specific Populations (8.4)].
Close5.13 Embryo-Fetal Toxicity
Based on its mechanism of action, BLINCYTO may cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to the fetus. Advise females of reproductive potential to use effective contraception during treatment with BLINCYTO and for 48 hours after the last dose [see Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions are described elsewhere in the labeling: Cytokine Release Syndrome [see Warnings and Precautions (5.1)] Neurological Toxicities, including ...
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Cytokine Release Syndrome [see Warnings and Precautions (5.1)]
- Neurological Toxicities, including Immune Effector Cell-Associated Neurotoxicity Syndrome [see Warnings and Precautions (5.2)]
- Infections [see Warnings and Precautions (5.3)]
- Tumor Lysis Syndrome [see Warnings and Precautions (5.4)]
- Neutropenia and Febrile Neutropenia [see Warnings and Precautions (5.5)]
- Effects on Ability to Drive and Use Machines [see Warnings and Precautions (5.6)]
- Elevated Liver Enzymes [see Warnings and Precautions (5.7)]
- Pancreatitis [see Warnings and Precautions (5.8)]
- Leukoencephalopathy [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of BLINCYTO in adult and pediatric patients one month and older with MRD-positive B-cell precursor ALL (n = 137), relapsed or refractory B-cell precursor ALL (n = 267), and Philadelphia chromosome-negative B-cell precursor ALL in consolidation (n = 165) was evaluated in clinical studies. The most common adverse reactions (≥ 20%) to BLINCYTO in this pooled population were pyrexia, infusion-related reactions, headache, infection, musculoskeletal pain, neutropenia, nausea, anemia, thrombocytopenia, and diarrhea.
MRD-positive B-cell Precursor ALL
The safety of BLINCYTO in patients with MRD-positive B-cell precursor ALL was evaluated in two single-arm clinical studies in which 137 adult patients were treated with BLINCYTO. The median age of the study population was 45 years (range: 18 to 77 years).
The most common adverse reactions (≥ 20%) were pyrexia, infusion-related reactions, headache, infections (pathogen unspecified), tremor, and chills. Serious adverse reactions were reported in 61% of patients. The most common serious adverse reactions (≥ 2%) included pyrexia, tremor, encephalopathy, aphasia, lymphopenia, neutropenia, overdose, device related infection, seizure, and staphylococcal infection. Adverse reactions of Grade 3 or higher were reported in 64% of patients. Discontinuation of therapy due to adverse reactions occurred in 17% of patients; neurologic events were the most frequently reported reasons for discontinuation. There were 2 fatal adverse reactions that occurred within 30 days of the end of BLINCYTO treatment (atypical pneumonia and subdural hemorrhage).
Table 6 summarizes the adverse reactions occurring at a ≥ 10% incidence for any grade or ≥ 5% incidence for Grade 3 or higher.
Table 6. Adverse Reactions Occurring at ≥ 10% Incidence for Any Grade or ≥ 5% Incidence for Grade 3 or Higher in BLINCYTO-treated Adult Patients with MRD-Positive B-cell Precursor ALL Adverse Reaction BLINCYTO
(N = 137)Any Grade*
n (%)Grade ≥ 3*
n (%)- *
- Grading based on NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
- †
- Neutropenia includes febrile neutropenia, neutropenia, and neutrophil count decreased.
- ‡
- Leukopenia includes leukopenia and white blood cell count decreased.
- §
- Thrombocytopenia includes platelet count decreased and thrombocytopenia.
- ¶
- Arrhythmia includes bradycardia, sinus arrhythmia, sinus bradycardia, sinus tachycardia, tachycardia and ventricular extrasystoles.
- #
- Pyrexia includes body temperature increased and pyrexia.
- Þ
- Infusion-related reaction is a composite term that includes the term infusion-related reaction and the following events occurring with the first 48 hours of infusion and the event lasted ≤ 2 days: cytokine release syndrome, eye swelling, hypertension, hypotension, myalgia, periorbital edema, pruritus generalized, pyrexia, and rash.
- ß
- Decreased immunoglobulins includes blood immunoglobulin A decreased, blood immunoglobulin G decreased, blood immunoglobulin M decreased, hypogammaglobulinemia, hypoglobulinemia, and immunoglobulins decreased.
- à
- Hypertransaminasemia includes alanine aminotransferase increased, aspartate aminotransferase increased, and hepatic enzyme increased.
- è
- May represent ICANS.
- ð
- Tremor includes essential tremor, intention tremor, and tremor.
- ø
- Encephalopathy includes cognitive disorder, depressed level of consciousness, disturbance in attention, encephalopathy, lethargy, leukoencephalopathy, memory impairment, somnolence, and toxic encephalopathy.
- ý
- Insomnia includes initial insomnia, insomnia, and terminal insomnia.
- £
- Rash includes dermatitis contact, eczema, erythema, rash, and rash maculopapular.
Blood and lymphatic system disorders Neutropenia† 21 (15) 21 (15) Leukopenia‡ 19 (14) 13 (9) Thrombocytopenia§ 14 (10) 8 (6) Cardiac disorders Arrhythmia¶ 17 (12) 3 (2) General disorders and administration site conditions Pyrexia# 125 (91) 9 (7) Chills 39 (28) 0 (0) Infections and infestations Infections - pathogen unspecified 53 (39) 11 (8) Injury, poisoning and procedural complications Infusion-related reactionÞ 105 (77) 7 (5) Investigations Decreased immunoglobulinsß 25 (18) 7 (5) Weight increased 14 (10) 1 (< 1) Hypertransaminasemiaà 13 (9) 9 (7) Musculoskeletal and connective tissue disorders Back pain 16 (12) 1 (< 1) Nervous system disorders Headacheè 54 (39) 5 (4) Tremorè,ð 43 (31) 6 (4) Aphasiaè 16 (12) 1 (< 1) Dizzinessè 14 (10) 1 (< 1) Encephalopathyè,ø 14 (10) 6 (4) Psychiatric disorders Insomniaè,ý 24 (18) 1 (< 1) Respiratory, thoracic and mediastinal disorders Cough 18 (13) 0 (0) Skin and subcutaneous tissue disorders Rash£ 22 (16) 1 (< 1) Vascular disorders Hypotension 19 (14) 1 (< 1) Additional adverse reactions in adult patients with MRD-positive ALL that did not meet the threshold criteria for inclusion in Table 6 were:
Blood and lymphatic system disorders: anemia
General disorders and administration site conditions: edema peripheral, pain, and chest pain (includes chest pain and musculoskeletal chest pain)
Hepatobiliary disorders: blood bilirubin increased
Immune system disorders: hypersensitivity and cytokine release syndrome
Infections and infestations: viral infectious disorders, bacterial infectious disorders, and fungal infectious disorders
Injury, poisoning and procedural complications: medication error and overdose (includes overdose and accidental overdose)
Investigations: blood alkaline phosphatase increased
Musculoskeletal and connective tissue disorders: pain in extremity and bone pain
Nervous system disorders: seizure (includes seizure and generalized tonic-clonic seizure), speech disorder, and hypoesthesia
Psychiatric disorders: confusional state, disorientation, and depression
Respiratory, thoracic and mediastinal disorders: dyspnea and productive cough
Vascular disorders: hypertension (includes blood pressure increased and hypertension) flushing (includes flushing and hot flush), and capillary leak syndrome
Relapsed or Refractory B-cell Precursor ALL
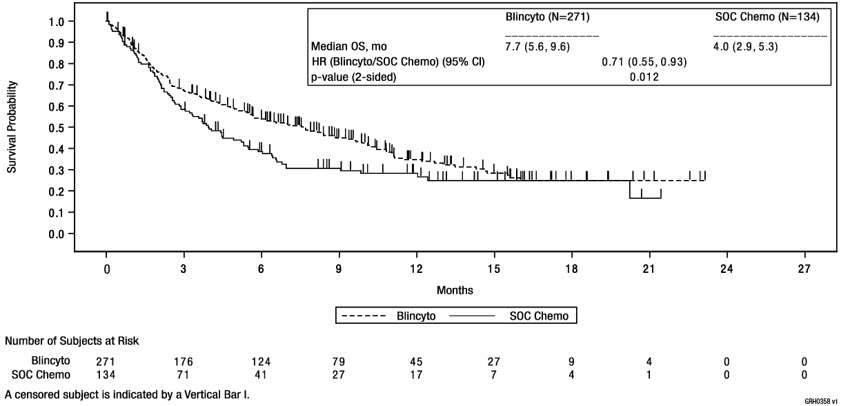
The safety of BLINCYTO was evaluated in a randomized, open-label, active-controlled clinical study (TOWER Study) in which 376 adult patients with Philadelphia chromosome-negative relapsed or refractory B-cell precursor ALL were treated with BLINCYTO (n = 267) or standard of care (SOC) chemotherapy (n = 109). The median age of BLINCYTO-treated patients was 37 years (range: 18 to 80 years), 60% were male, 84% were White, 7% Asian, 2% were Black or African American, 2% were American Indian or Alaska Native, and 5% were Multiple/Other.
The most common adverse reactions (≥ 20%) in the BLINCYTO arm were infections (bacterial and pathogen unspecified), pyrexia, headache, infusion-related reactions, anemia, febrile neutropenia, thrombocytopenia, and neutropenia. Serious adverse reactions were reported in 62% of patients. The most common serious adverse reactions (≥ 2%) included febrile neutropenia, pyrexia, sepsis, pneumonia, overdose, septic shock, CRS, bacterial sepsis, device related infection, and bacteremia. Adverse reactions of Grade 3 or higher were reported in 87% of patients. Discontinuation of therapy due to adverse reactions occurred in 12% of patients treated with BLINCYTO; neurologic events and infections were the most frequently reported reasons for discontinuation of treatment due to an adverse reaction. Fatal adverse events occurred in 16% of patients. The majority of the fatal events were infections.
The adverse reactions occurring at a ≥ 10% incidence for any grade or ≥ 5% incidence for Grade 3 or higher in the BLINCYTO-treated patients in first cycle of therapy are summarized in Table 7.
Table 7. Adverse Reactions Occurring at ≥ 10% Incidence for Any Grade or ≥ 5% Incidence for Grade 3 or Higher in BLINCYTO-Treated Patients in First Cycle of Therapy for Adult Patients with Relapsed or Refractory B-cell Precursor ALL (TOWER Study) Adverse Reaction BLINCYTO
(N = 267)Standard of Care (SOC) Chemotherapy
(N = 109)Any Grade*
n (%)Grade ≥ 3*
n (%)Any Grade*
n (%)Grade ≥ 3*
n (%)- *
- Grading based on NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
- †
- Neutropenia includes agranulocytosis, febrile neutropenia, neutropenia, and neutrophil count decreased.
- ‡
- Anemia includes anemia and hemoglobin decreased.
- §
- Thrombocytopenia includes platelet count decreased and thrombocytopenia.
- ¶
- Leukopenia includes leukopenia and white blood cell count decreased.
- #
- Arrhythmia includes arrhythmia, atrial fibrillation, atrial flutter, bradycardia, sinus bradycardia, sinus tachycardia, supraventricular tachycardia, and tachycardia.
- Þ
- Edema includes face edema, fluid retention, edema, edema peripheral, peripheral swelling, and swelling face.
- ß
- Cytokine release syndrome includes cytokine release syndrome and cytokine storm.
- à
- Infusion-related reaction is a composite term that includes the term infusion-related reaction and the following events occurring with the first 48 hours of infusion and the event lasted ≤ 2 days: pyrexia, cytokine release syndrome, hypotension, myalgia, acute kidney injury, hypertension, and rash erythematous.
- è
- Hypertransaminasemia includes alanine aminotransferase increased, aspartate aminotransferase increased, hepatic enzyme increased, and transaminases increased.
- ð
- May represent ICANS.
- ø
- Rash includes erythema, rash, rash erythematous, rash generalized, rash macular, rash maculo-papular, rash pruritic, skin exfoliation, and toxic skin eruption.
Blood and lymphatic system disorders Neutropenia† 84 (31) 76 (28) 67 (61) 61 (56) Anemia‡ 68 (25) 52 (19) 45 (41) 37 (34) Thrombocytopenia§ 57 (21) 47 (18) 42 (39) 40 (37) Leukopenia¶ 21 (8) 18 (7) 9 (8) 9 (8) Cardiac disorders Arrhythmia# 37 (14) 5 (2) 18 (17) 0 (0) General disorders and administration site conditions Pyrexia 147 (55) 15 (6) 43 (39) 4 (4) EdemaÞ 48 (18) 3 (1) 20 (18) 1 (1) Immune system disorders Cytokine release syndromeß 37 (14) 8 (3) 0 (0) 0 (0) Infections and infestations Infections - pathogen unspecified 74 (28) 40 (15) 50 (46) 35 (32) Bacterial infectious disorders 38 (14) 19 (7) 35 (32) 21 (19) Viral infectious disorders 30 (11) 4 (1) 14 (13) 0 (0) Fungal infectious disorders 27 (10) 13 (5) 15 (14) 9 (8) Injury, poisoning and procedural complications Infusion-related reactionà 79 (30) 9 (3) 9 (8) 1 (1) Investigations Hypertransaminasemiaè 40 (15) 22 (8) 13 (12) 7 (6) Nervous system disorders Headacheð 61 (23) 1 (< 1) 30 (28) 3 (3) Skin and subcutaneous tissue disorders Rashø 31 (12) 2 (1) 21 (19) 0 (0) Selected laboratory abnormalities worsening from baseline Grade 0-2 to treatment-related maximal Grade 3-4 in first cycle of therapy are shown in Table 8.
Table 8. Selected Laboratory Abnormalities Worsening from Baseline Grade 0-2 to Treatment-related Maximal Grade 3-4* in First Cycle of Therapy for Adult Patients with Relapsed or Refractory B-cell Precursor ALL (TOWER Study) BLINCYTO
Grade 3 or 4 (%)SOC Chemotherapy
Grade 3 or 4 (%)- *
- Includes only patients who had both baseline and at least one laboratory measurement during first cycle of therapy available.
Hematology Decreased lymphocyte count 80 83 Decreased white blood cell count 53 97 Decreased hemoglobin 29 43 Decreased neutrophil count 57 68 Decreased platelet count 47 85 Chemistry Increased ALT 11 11 Increased bilirubin 5 4 Increased AST 8 4 Other important adverse reactions from pooled relapsed or refractory B-cell precursor ALL studies were:
Blood and lymphatic system disorders: lymphadenopathy, hematophagic histiocytosis, and leukocytosis (includes leukocytosis and white blood cell count increased)
General disorders and administration site conditions: chills, chest pain (includes chest discomfort, chest pain, musculoskeletal chest pain, and non-cardiac chest pain), pain, body temperature increased, hyperthermia, and systemic inflammatory response syndrome
Hepatobiliary disorders: hyperbilirubinemia (includes blood bilirubin increased and hyperbilirubinemia)
Immune system disorders: hypersensitivity (includes hypersensitivity, anaphylactic reaction, angioedema, dermatitis allergic, drug eruption, drug hypersensitivity, erythema multiforme, and urticaria)
Injury, poisoning and procedural complications: medication error and overdose (includes overdose, medication error, and accidental overdose)
Investigations: weight increased, decreased immunoglobulins (includes immunoglobulins decreased, blood immunoglobulin A decreased, blood immunoglobulin G decreased, blood immunoglobulin M decreased, and hypogammaglobulinemia), blood alkaline phosphatase increased, and hypertransaminasemia
Metabolism and nutrition disorders: tumor lysis syndrome
Musculoskeletal and connective tissue disorders: back pain, bone pain, and pain in extremity
Nervous system disorders: tremor (resting tremor, intention tremor, essential tremor, and tremor), altered state of consciousness (includes altered state of consciousness, depressed level of consciousness, disturbance in attention, lethargy, mental status changes, stupor, and somnolence), dizziness, memory impairment, seizure (includes seizure, and atonic seizure), aphasia, cognitive disorder, speech disorder, hypoesthesia, encephalopathy, paresthesia, and cranial nerve disorders (trigeminal neuralgia, trigeminal nerve disorder, sixth nerve paralysis, cranial nerve disorder, facial nerve disorder, and facial paresis)
Psychiatric disorders: insomnia, disorientation, confusional state, and depression (includes depressed mood, depression, suicidal ideation, and completed suicide)
Respiratory, thoracic and mediastinal disorders: dyspnea (includes acute respiratory failure, dyspnea, dyspnea exertional, respiratory failure, respiratory distress, bronchospasm, bronchial hyperreactivity, tachypnea, and wheezing), cough, and productive cough
Vascular disorders: hypotension (includes blood pressure decreased, hypotension, hypovolemic shock, and circulatory collapse), hypertension (includes blood pressure increased, hypertension, and hypertensive crisis), flushing (includes flushing and hot flush), and capillary leak syndrome
B-cell Precursor ALL in the Consolidation Phase
Study E1910
The safety of a consolidation regimen comprised of multiple cycles of BLINCYTO monotherapy in addition to multiple cycles of chemotherapy (BLINCYTO arm) was evaluated in a randomized trial in adult patients with newly diagnosed Philadelphia chromosome-negative B-cell precursor ALL (Study E1910) [NCT02003222] [see Clinical Studies (14.3)] which included 111 patients treated in the BLINCYTO arm and 112 patients treated in the chemotherapy alone arm. In the BLINCYTO arm, the median (range) of cycles was 8 (1-8) (4 cycles of BLINCYTO and 4 cycles of chemotherapy). In the chemotherapy alone arm, the median (range) of cycles was 4 (1-4).
Fatal adverse reactions occurred in 2 patients (2%) during BLINCYTO cycles and were due to infection (n = 1) and coagulopathy (n = 1). Permanent discontinuation of BLINCYTO due to an adverse reaction occurred in 2% of patients. Dosage interruptions of BLINCYTO due to an adverse reaction occurred in 5% of patients. Dose reductions of BLINCYTO due to an adverse reaction occurred in 28% of patients.
The most common (≥ 20%) adverse reactions during consolidation cycles in the BLINCYTO arm were neutropenia, thrombocytopenia, anemia, leukopenia, headache, infection, nausea, lymphopenia, diarrhea, musculoskeletal pain, and tremor. The adverse reactions occurring at a difference between arms in incidence of ≥ 10% for All Grades or ≥ 5% for Grade 3 or higher are summarized in Table 9.
Table 9. Adverse Reactions with a Difference Between Arms of ≥ 10% for Any Grade or ≥ 5% for Grade 3 or 4 during Consolidation (Study E1910) Consolidation Consisting of Adverse Reaction BLINCYTO Cycles + Chemotherapy Cycles
(n = 111)Chemotherapy Cycles Alone
(n = 112)All Grades
(%)*Grade 3 or 4
(%)All Grades
(%)Grade 3 or 4
(%)- *
- Includes the following fatal adverse reaction: infection (n = 1).
- †
- Other related adverse reactions included:
- ‡
- Nausea: vomiting;
- §
- Cytokine release syndrome: capillary leak syndrome;
- ¶
- Musculoskeletal pain: pain in extremity, back pain, arthralgia, myalgia, neck pain, flank pain, bone pain, non-cardiac chest pain;
- #
- May represent ICANS.
- Þ
- Aphasia: dysarthria.
Blood and lymphatic system disorders Neutropenia† 82 77 89 89 Thrombocytopenia† 75 57 75 71 Anemia 59 29 50 38 Leukopenia† 43 41 57 56 Lymphopenia† 32 30 25 23 Febrile neutropenia 19 19 25 25 Gastrointestinal disorders Nausea‡ 32 5 22 4 Diarrhea† 29 3 15 3 Immune system disorders Cytokine release syndrome§ 16 4 0 0 Infections and infestations Infection – pathogen unspecified 35 31 22 21 Musculoskeletal and connective tissue disorders Musculoskeletal pain¶ 23 5 5 4 Nervous system disorders Headache# 41 5 30 5 Tremor# 23 3 3 0 AphasiaÞ,# 10 8 0 0 Vascular disorders Hypertension 12 10 5 3 Study 20120215
The safety of BLINCYTO as the 3rd cycle of the consolidation phase was evaluated in a randomized, open-label study (Study 20120215) following induction and two cycles of consolidation chemotherapy in pediatric and young adult patients with high-risk first-relapsed B-cell precursor ALL [see Clinical Studies (14.3)]. The study included 54 patients treated with one cycle of BLINCYTO and 52 patients treated with one cycle of chemotherapy.
Serious adverse reactions occurred in 28% of patients who received BLINCYTO. Permanent discontinuation of BLINCYTO due to an adverse reaction occurred in 4% of patients. Adverse reactions that led to discontinuation included nervous system disorder and seizure. Dosage interruptions of BLINCYTO due to an adverse reaction occurred in 11% of patients. Adverse reactions which required dosage interruption in > 2% of patients included nervous system disorder.
The most common (≥ 20%) adverse reactions in the BLINCYTO arm were pyrexia, nausea, headache, rash, hypogammaglobulinemia, and anemia. The adverse reactions occurring at a difference of ≥ 10% incidence for any grade or at a difference of ≥ 5% incidence for Grade 3 or 4 between the BLINCYTO arm and chemotherapy arm are summarized in Table 10.
Table 10. Adverse Reactions with a Difference Between Arms of ≥ 10% for Any Grade or ≥ 5% for Grade 3 or 4 during Consolidation Cycle 3 (Study 20120215) Adverse Reaction BLINCYTO
(n = 54)Chemotherapy
(n = 52)All Grades
(%)Grade 3 or 4
(%)All Grades
(%)Grade 3 or 4
(%)- *
- Other related adverse reactions included:
- †
- Nausea: vomiting;
- ‡
- Stomatitis: mouth ulceration, mucosal inflammation;
- §
- Liver function test abnormal: alanine aminotransferase increased, aspartate aminotransferase increased, gamma-glutamyltransferase increased, hypertransaminasemia;
- ¶
- Musculoskeletal pain: back pain, pain in extremity, bone pain;
- #
- May represent ICANS.
- Þ
- Hemorrhage: Epistaxis, petechiae, hemarthrosis, hematoma, hematuria.
Blood and lymphatic system disorders Anemia* 24 15 46 42 Neutropenia* 19 17 35 31 Thrombocytopenia* 15 15 39 35 Febrile neutropenia 2 2 25 25 Gastrointestinal disorders Nausea† 43 2 31 2 Abdominal pain* 13 0 23 2 Stomatitis‡ 11 4 60 29 General disorders and administration site conditions Pyrexia 76 6 19 0 Hepatobiliary disorders Liver function test abnormal§ 9 6 27 17 Immune system disorders Hypogammaglobulinemia* 24 2 12 2 Infections and infestations Infection – pathogen unspecified 13 6 29 10 Musculoskeletal and connective tissue disorders Musculoskeletal pain¶ 9 0 29 2 Nervous system disorders Headache# 37 0 15 0 Skin and subcutaneous disorders Rash* 22 2 12 0 Vascular disorders HemorrhageÞ 11 2 23 6 Close6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of BLINCYTO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Fatal pancreatitis in patients receiving BLINCYTO in combination with dexamethasone.
-
7 DRUG INTERACTIONSNo formal drug interaction studies have been conducted with BLINCYTO. Initiation of BLINCYTO treatment causes transient release of cytokines that may suppress CYP450 enzymes. The highest drug-drug ...
No formal drug interaction studies have been conducted with BLINCYTO. Initiation of BLINCYTO treatment causes transient release of cytokines that may suppress CYP450 enzymes. The highest drug-drug interaction risk is during the first 9 days of the first cycle and the first 2 days of the second cycle in patients who are receiving concomitant CYP450 substrates, particularly those with a narrow therapeutic index. In these patients, monitor for toxicity (e.g., warfarin) or drug concentrations (e.g., cyclosporine). Adjust the dose of the concomitant drug as needed [see Clinical Pharmacology (12.2, 12.3)].
Close -
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Based on its mechanism of action, BLINCYTO may cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available ...
8.1 Pregnancy
Risk Summary
Based on its mechanism of action, BLINCYTO may cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data on the use of BLINCYTO in pregnant women to evaluate for a drug-associated risk. In animal reproduction studies, a murine surrogate molecule administered to pregnant mice crossed the placental barrier (see Data).
Blinatumomab causes T-cell activation and cytokine release; immune activation may compromise pregnancy maintenance. In addition, based on expression of CD19 on B-cells and the finding of B-cell depletion in non-pregnant animals, blinatumomab can cause B-cell lymphocytopenia in infants exposed to blinatumomab in-utero. Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Due to the potential for B-cell lymphocytopenia in infants following exposure to BLINCYTO in utero, the infant's B lymphocytes should be monitored before the initiation of live virus vaccination [see Warnings and Precautions (5.11)].
Data
Animal Data
Animal reproduction studies have not been conducted with blinatumomab. In embryo-fetal developmental toxicity studies, a murine surrogate molecule was administered intravenously to pregnant mice during the period of organogenesis. The surrogate molecule crossed the placental barrier and did not cause embryo-fetal toxicity or teratogenicity. The expected depletions of B and T cells were observed in the pregnant mice, but hematological effects were not assessed in fetuses.
8.2 Lactation
Risk Summary
There is no information regarding the presence of blinatumomab in human milk, the effects on the breastfed infant, or the effects on milk production. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in breastfed infants from BLINCYTO, including B-cell lymphocytopenia, advise patients not to breastfeed during treatment with BLINCYTO and for 48 hours after the last dose.
8.3 Females and Males of Reproductive Potential
BLINCYTO may cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
8.4 Pediatric Use
The safety and efficacy of BLINCYTO in pediatric patients less than 1 month of age have not been established for any indication [see Indications and Usage (1)].
Minimal Residual Disease (MRD)-Positive B-cell Precursor ALL
The safety and efficacy of BLINCYTO for the treatment of CD19-positive B-cell precursor acute lymphoblastic leukemia (ALL) in first or second complete remission with minimal residual disease (MRD) greater than or equal to 0.1% have been established in pediatric patients one month and older. Use of BLINCYTO is supported by evidence from two randomized, controlled trials (Study AALL1331, NCT02101853 and Study 20120215, NCT02393859) [see Clinical Studies (14.3)] in pediatric patients with first relapsed B-cell precursor ALL. Both studies included pediatric patients with MRD-positive B-cell precursor ALL. The studies included pediatric patients treated with BLINCYTO in the following age groups: 6 infants (1 month up to less than 2 years), 165 children (2 years up to less than 12 years), and 70 adolescents (12 years to less than 17 years). In general, the adverse reactions in BLINCYTO-treated pediatric patients were similar in type to those seen in adult patients with MRD-positive ALL [see Adverse Reactions (6.1)], and no differences in safety were observed between the different pediatric age subgroups.
Relapsed or Refractory B-cell Precursor ALL
The safety and efficacy of BLINCYTO have been established in pediatric patients one month and older with relapsed or refractory B-cell precursor ALL. Use of BLINCYTO is supported by a single-arm trial in pediatric patients with relapsed or refractory B-cell precursor ALL. This study included pediatric patients in the following age groups: 10 infants (1 month up to less than 2 years), 40 children (2 years up to less than 12 years), and 20 adolescents (12 years to less than 18 years). No differences in efficacy were observed between the different age subgroups [see Clinical Studies (14.2)].
In general, the adverse reactions in BLINCYTO-treated pediatric patients with relapsed or refractory ALL were similar in type to those seen in adult patients with relapsed or refractory B-cell precursor ALL [see Adverse Reactions (6.1)]. Adverse reactions that were observed more frequently (≥ 10% difference) in the pediatric population compared to the adult population were pyrexia (80% vs. 61%), hypertension (26% vs. 8%), anemia (41% vs. 24%), infusion-related reaction (49% vs. 34%), thrombocytopenia (34% vs. 21%), leukopenia (24% vs. 11%), and weight increased (17% vs. 6%).
In pediatric patients less than 2 years old (infants) with relapsed or refractory ALL, the incidence of neurologic toxicities was not significantly different than for the other age groups, but its manifestations were different; the only event terms reported were agitation, headache, insomnia, somnolence, and irritability. Infants also had an increased incidence of hypokalemia (50%) compared to other pediatric age cohorts (15-20%) or adults (17%).
B-cell Precursor ALL in the Consolidation Phase
The safety and efficacy of BLINCYTO for the treatment of Philadelphia-chromosome negative B-cell precursor ALL in the consolidation phase have been established in pediatric patients one month and older. Use of BLINCYTO for this indication is supported by extrapolation from a randomized controlled study in adults (Study E1910, NCT02003222) and evidence from two randomized, controlled studies in pediatric patients (Study 20120215 and Study AALL1331) [see Adverse Reactions (6.1), Use in Specific Populations (8.4), Clinical Pharmacology (12.3), and Clinical Studies (14.3)].
Benzyl Alcohol Toxicity in Neonates
Serious and fatal adverse reactions, including "gasping syndrome," can occur in very low birth weight (VLBW) neonates born weighing less than 1500 g, and early preterm neonates (infants born less than 34 weeks gestational age) treated with benzyl alcohol-preserved drugs intravenously. The "gasping syndrome" is characterized by central nervous system depression, metabolic acidosis, and gasping respirations. In these cases, benzyl alcohol dosages of 99 to 234 mg/kg/day produced high concentrations of benzyl alcohol and its metabolite in the blood and urine (blood concentration of benzyl alcohol were 0.61 to 1.378 mmol/L). Additional adverse reactions included gradual neurological deterioration, seizures, intracranial hemorrhage, hematologic abnormalities, skin breakdown, hepatic and renal failure, hypotension, bradycardia, and cardiovascular collapse. The minimum amount of benzyl alcohol at which serious adverse reactions may occur in neonates is not known [see Warnings and Precautions (5.12)].
Use the preservative-free formulations of BLINCYTO where possible in neonates. When prescribing BLINCYTO (with preservative) in neonatal patients, consider the combined daily metabolic load of benzyl alcohol from all sources including BLINCYTO (with preservative). The BLINCYTO 72-Hour bag (with preservative) and 96-Hour bag (with preservative) contain 2.5 mg of benzyl alcohol per mL, and the 7-Day bag (with preservative) contains 7.4 mg of benzyl alcohol per mL. The administration of BLINCYTO as a 72-hour, 96-hour, and 7-day infusion is not recommended for patients weighing less than 5.4 kg [see Warnings and Precautions (5.12)].
Benzyl alcohol administration may contribute to metabolic acidosis in pediatric patients, particularly those with immaturity of the metabolic pathway for alcohol, or those with underlying conditions or receiving concomitant medications that could predispose to acid base imbalance. Monitor these patients during use of BLINCYTO (with preservative) for new or worsening metabolic acidosis.
Close8.5 Geriatric Use
There were 158 (7%) patients 65 years and older in clinical studies of BLINCYTO for patients with MRD positive, CD19-positive B-cell precursor ALL in first or second complete remission, relapsed or refractory CD19-positive B-cell precursor ALL, and CD19-positive, Philadelphia-chromosome negative B-cell precursor ALL in the consolidation phase. Of the total number of BLINCYTO-treated patients in these studies, 123 (8%) were 65 years of age and older and 21 (1%) were 75 years of age or older. No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients. However, elderly patients experienced a higher rate of serious infections and neurological toxicities, including cognitive disorder, encephalopathy, and confusion [see Warnings and Precautions (5.2, 5.3)].
-
10 OVERDOSAGEOverdoses have been observed, including one adult patient who received 133-fold the recommended therapeutic dose of BLINCYTO delivered over a short duration. In the dose evaluation phase of a ...
Overdoses have been observed, including one adult patient who received 133-fold the recommended therapeutic dose of BLINCYTO delivered over a short duration.
In the dose evaluation phase of a study in pediatric and adolescent patients with relapsed or refractory B-cell precursor ALL, one patient experienced a fatal cardiac failure event in the setting of life-threatening cytokine release syndrome (CRS) at a 30 mcg/m2/day (higher than the maximum tolerated/recommended) dose [see Warnings and Precautions (5.1) and Adverse Reactions (6)].
Overdoses resulted in adverse reactions, which were consistent with the reactions observed at the recommended dosage and included fever, tremors, and headache. In the event of overdose, interrupt the infusion, monitor the patient for signs of adverse reactions, and provide supportive care [see Warnings and Precautions (5.10)]. Consider re-initiation of BLINCYTO at the recommended dosage when all adverse reactions have resolved and no earlier than 12 hours after interruption of the infusion [see Dosage and Administration (2.1, 2.2 and 2.3)].
Close -
11 DESCRIPTIONBlinatumomab is a bispecific CD19-directed CD3 T-cell engager. Blinatumomab is produced in Chinese hamster ovary cells. It consists of 504 amino acids and has a molecular weight of approximately ...
Blinatumomab is a bispecific CD19-directed CD3 T-cell engager. Blinatumomab is produced in Chinese hamster ovary cells. It consists of 504 amino acids and has a molecular weight of approximately 54 kilodaltons.
Each BLINCYTO package contains one vial BLINCYTO and one vial IV Solution Stabilizer.
BLINCYTO (blinatumomab) for injection is supplied in a single-dose vial as a sterile, preservative-free, white to off-white lyophilized powder for intravenous use. Each single-dose vial of BLINCYTO contains 35 mcg blinatumomab, citric acid monohydrate (3.35 mg), lysine hydrochloride (23.23 mg), polysorbate 80 (0.64 mg), trehalose dihydrate (95.5 mg), and sodium hydroxide to adjust pH to 7.0. After reconstitution with 3 mL of preservative-free Sterile Water for Injection, USP, the resulting concentration is 12.5 mcg/mL blinatumomab.
IV Solution Stabilizer is supplied in a single-dose vial as a sterile, preservative-free, colorless to slightly yellow, clear solution. Each single-dose vial of IV Solution Stabilizer contains citric acid monohydrate (52.5 mg), lysine hydrochloride (2283.8 mg), polysorbate 80 (10 mg), sodium hydroxide to adjust pH to 7.0, and water for injection.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Blinatumomab is a bispecific CD19-directed CD3 T-cell engager that binds to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface ...
12.1 Mechanism of Action
Blinatumomab is a bispecific CD19-directed CD3 T-cell engager that binds to CD19 expressed on the surface of cells of B-lineage origin and CD3 expressed on the surface of T-cells. It activates endogenous T-cells by connecting CD3 in the T-cell receptor (TCR) complex with CD19 on benign and malignant B-cells. Blinatumomab mediates the formation of a synapse between the T-cell and the tumor cell, upregulation of cell adhesion molecules, production of cytolytic proteins, release of inflammatory cytokines, and proliferation of T-cells, which result in redirected lysis of CD19+ cells.
12.2 Pharmacodynamics
During the continuous intravenous infusion over 4 weeks, the pharmacodynamic response was characterized by T-cell activation and initial redistribution, reduction in peripheral B-cells, and transient cytokine elevation.
Peripheral T-cell redistribution (i.e., T-cell adhesion to blood vessel endothelium and/or transmigration into tissue) occurred after start of BLINCYTO infusion or dose escalation. T-cell counts initially declined within 1 to 2 days and then returned to baseline levels within 7 to 14 days in the majority of patients. Increase of T-cell counts above baseline (T-cell expansion) was observed in few patients.
Peripheral B-cell counts decreased to less than or equal to 10 cells/microliter during the first treatment cycle at doses ≥ 5 mcg/m2/day or ≥ 9 mcg/day in the majority of patients. No recovery of peripheral B-cell counts was observed during the 2-week BLINCYTO-free period between treatment cycles. Incomplete depletion of B-cells occurred at doses of 0.5 mcg/m2/day and 1.5 mcg/m2/day and in a few patients at higher doses.
Cytokines including IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, TNF-α, and IFN-γ were measured, and IL-6, IL-10, and IFN-γ were elevated. The highest elevation of cytokines was observed in the first 2 days following start of BLINCYTO infusion. The elevated cytokine levels returned to baseline within 24 to 48 hours during the infusion. In subsequent treatment cycles, cytokine elevation occurred in fewer patients with lesser intensity compared to the initial 48 hours of the first treatment cycle.
12.3 Pharmacokinetics
The pharmacokinetics of blinatumomab appear linear over a dose range from 5 to 90 mcg/m2/day (approximately equivalent to 9 to 162 mcg/day) in adult patients. Following continuous intravenous infusion, the steady-state serum concentration (Css) was achieved within a day and remained stable over time. The increase in mean Css values was approximately proportional to the dose in the range tested. At the clinical doses of 9 mcg/day and 28 mcg/day for the treatment of relapsed or refractory ALL, the mean (SD) Css was 228 (356) pg/mL and 616 (537) pg/mL, respectively. The pharmacokinetics of blinatumomab in adult patients with MRD-positive B-cell precursor ALL and in adult patients with B-cell precursor ALL in the consolidation phase were similar to adult patients with relapsed or refractory ALL.
Distribution
The estimated mean (SD) volume of distribution based on terminal phase (Vz) was 5.27 (4.37) L with continuous intravenous infusion of blinatumomab.
Elimination
The estimated mean (SD) systemic clearance with continuous intravenous infusion in patients receiving blinatumomab in clinical studies was 3.10 (2.94) L/hour. The mean (SD) half-life was 2.20 (1.34) hours. Negligible amounts of blinatumomab were excreted in the urine at the tested clinical doses.
Specific Populations
There were no clinically meaningful differences in the pharmacokinetics of blinatumomab based on age (0.6 to 80 years of age), sex, race (72% White, 17% Asian, 3% Black), ethnicity, Philadelphia chromosome status or mild (total bilirubin ≤ upper limit of normal [ULN] and AST > ULN or total bilirubin > 1 to 1.5 × ULN and any AST) or moderate hepatic impairment (total bilirubin > 1.5 to 3 × ULN and any AST). The effect of other races or severe hepatic impairment (total bilirubin > 3 × ULN, any AST) on the pharmacokinetics of blinatumomab is unknown. Body surface area (0.4 to 2.9 m2) influences the pharmacokinetics of blinatumomab, supporting BSA-based dosing in patients < 45 kg.
Pediatric Patients
The pharmacokinetics of blinatumomab appear linear over a dose range from 5 to 30 mcg/m2/day in pediatric patients. At the recommended doses of 5 and 15 mcg/m2/day for the treatment of relapsed or refractory B-cell precursor ALL, the mean (SD) steady-state concentration (Css) values were 162 (179) and 533 (392) pg/mL, respectively. The pharmacokinetics of blinatumomab in pediatric patients with MRD-positive B-cell precursor ALL and in pediatric patients with B-cell precursor ALL in the consolidation phase were similar to pediatric patients with relapsed or refractory ALL.
In all pediatric patients with ALL, the estimated mean (SD) volume of distribution (Vz), clearance (CL), and terminal half-life (t1/2,z) in Cycle 1 were 4.14 (3.32) L/m2, 1.65 (1.62) L/hour/m2, and 2.14 (1.44) hours, respectively.
The steady-state concentrations of blinatumomab were comparable in adult and pediatric patients at the equivalent dose levels based on BSA-based regimens.
Patients with Renal Impairment
Pharmacokinetic analyses showed an approximately 2-fold difference in mean blinatumomab clearance values between patients with moderate renal impairment (CrCL ranging from 30 to 59 mL/min, N = 49) and normal renal function (CrCL more than 90 mL/min, N = 674). However, high interpatient variability was discerned (CV% up to 98.4%), and clearance values in renal impaired patients were essentially within the range observed in patients with normal renal function. There is no information available in patients with severe renal impairment (CrCL 15-29 mL/min) or patients on hemodialysis.
Drug Interaction Studies
Transient elevation of cytokines may suppress CYP450 enzyme activities [see Drug Interactions (7) and Clinical Pharmacology (12.2)].
Close12.6 Immunogenicity
The observed incidence of anti-drug antibody is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibody in the studies described below with the incidence of anti-drug antibodies in other studies, including those of BLINCYTO.
The immunogenicity of BLINCYTO has been evaluated using either an electrochemiluminescence detection technology (ECL) or an enzyme-linked immunosorbent assay (ELISA) screening immunoassay for the detection of binding anti-blinatumomab antibodies. For patients whose sera tested positive in the screening immunoassay, an in vitro biological assay was performed to detect neutralizing antibodies.
In clinical studies, less than 2% of patients treated with BLINCYTO tested positive for binding anti-blinatumomab antibodies. Of patients who developed anti-blinatumomab antibodies, 7 out of 9 (78%) had in vitro neutralizing activity. Anti-blinatumomab antibody formation may affect pharmacokinetics of BLINCYTO.
Overall, the totality of clinical evidence supports the finding that anti-blinatumomab antibodies are not suggestive of any clinical impact on the safety or effectiveness of BLINCYTO.
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No carcinogenicity or genotoxicity studies have been conducted with blinatumomab. No studies have been conducted to evaluate the ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity or genotoxicity studies have been conducted with blinatumomab.
No studies have been conducted to evaluate the effects of blinatumomab on fertility. A murine surrogate molecule had no adverse effects on male and female reproductive organs in a 13-week repeat-dose toxicity study in mice.
-
14 CLINICAL STUDIES14.1 MRD-positive B-cell Precursor ALL - BLAST Study - The efficacy of BLINCYTO was evaluated in an open-label, multicenter, single-arm study (BLAST Study) [NCT01207388] that included patients ...
14.1 MRD-positive B-cell Precursor ALL
BLAST Study
The efficacy of BLINCYTO was evaluated in an open-label, multicenter, single-arm study (BLAST Study) [NCT01207388] that included patients who were ≥ 18 years of age, had received at least 3 chemotherapy blocks of standard ALL therapy, were in hematologic complete remission (defined as < 5% blasts in bone marrow, absolute neutrophil count > 1 Gi/L, platelets > 100 Gi/L) and had MRD at a level of ≥ 0.1% using an assay with a minimum sensitivity of 0.01%. BLINCYTO was administered at a constant dose of 15 mcg/m2/day (equivalent to the recommended dosage of 28 mcg/day) intravenously for all treatment cycles. Patients received up to 4 cycles of treatment. Dose adjustment was possible in case of adverse events.
The treated population included 86 patients in first or second hematologic complete remission (CR1 or CR2). The demographics and baseline characteristics are shown in Table 11. The median number of treatment cycles was 2 (range: 1 to 4). Following treatment with BLINCYTO, 45 out of 61 (73.8%) patients in CR1 and 14 out of 25 (56.0%) patients in CR2 underwent allogeneic hematopoietic stem cell transplantation in continuous hematologic complete remission.
Table 11. Demographics and Baseline Characteristics in BLAST Study Characteristics BLINCYTO
(N = 86)- *
- Assessed centrally using an assay with minimum sensitivity of 0.01%.
Age Median, years (min, max) 43 (18, 76) ≥ 65 years, n (%) 10 (12) Males, n (%) 50 (58) Race, n (%) Asian 1 (1) Other (mixed) 0 (0) White 76 (88) Unknown 9 (11) Philadelphia chromosome disease status, n (%) Positive 1 (1) Negative 85 (99) Relapse history, n (%) Patients in 1st CR 61 (71) Patients in 2nd CR 25 (29) MRD level at baseline*, n (%) ≥ 10% 7 (8) ≥ 1% and < 10% 34 (40) ≥ 0.1% and < 1% 45 (52) Efficacy was based on achievement of undetectable MRD within one cycle of BLINCYTO treatment and hematological relapse-free survival (RFS). The assay used to assess MRD response had a sensitivity of 0.01% for 6 patients and ≤ 0.005% for 80 patients. Overall, undetectable MRD was achieved by 70 patients (81.4%: 95% CI: 71.6%, 89.0%). The median hematological RFS was 22.3 months. Table 12 shows the MRD response and hematological RFS by remission number.
Table 12. Efficacy Results in Patients ≥ 18 Years of Age with MRD-positive B-cell Precursor ALL (BLAST Study) Patients in CR1
(n = 61)Patients in CR2
(n = 25)- *
- Complete MRD response was defined as the absence of detectable MRD confirmed in an assay with minimum sensitivity of 0.01%
- †
- Relapse was defined as either hematological or extramedullary relapse, secondary leukemia, or death due to any cause; Includes time after transplantation; Kaplan-Meier estimate
Complete MRD response*, n (%),
[95% CI]52 (85.2)
[73.8, 93.0]18 (72.0)
[50.6, 87.9]Median hematological relapse-free survival† in months (range) 35.2
(0.4, 53.5)12.3
(0.7, 42.3)Undetectable MRD was achieved by 65 of 80 patients (81.3%: 95% CI: 71.0%, 89.1%) with an assay sensitivity of at least 0.005%. The estimated median hematological RFS among the 80 patients using the higher sensitivity assay was 24.2 months (95% CI: 17.9, NE).
14.2 Relapsed/Refractory B-cell Precursor ALL
TOWER Study
The efficacy of BLINCYTO was compared to standard of care (SOC) chemotherapy in a randomized, open-label, multicenter study (TOWER Study) [NCT02013167]. Eligible patients were ≥ 18 years of age with relapsed or refractory B-cell precursor ALL [> 5% blasts in the bone marrow and refractory to primary induction therapy or refractory to last therapy, untreated first relapse with first remission duration < 12 months, untreated second or later relapse, or relapse at any time after allogeneic hematopoietic stem cell transplantation (alloHSCT)]. BLINCYTO was administered at 9 mcg/day on Days 1-7 and 28 mcg/day on Days 8-28 for Cycle 1, and 28 mcg/day on Days 1-28 for Cycles 2-5 in 42-day cycles and for Cycles 6-9 in 84-day cycles. Dose adjustment was possible in case of adverse events. SOC chemotherapy included fludarabine, cytarabine arabinoside, and granulocyte colony-stimulating factor (FLAG); high-dose cytarabine arabinoside (HiDAC); high-dose methotrexate- (HDMTX) based combination; or clofarabine/clofarabine-based regimens.
There were 405 patients randomized 2:1 to receive BLINCYTO or investigator-selected SOC chemotherapy. Randomization was stratified by age (< 35 years vs. ≥ 35 years of age), prior salvage therapy (yes vs. no), and prior alloHSCT (yes vs. no) as assessed at the time of consent. The demographics and baseline characteristics were well-balanced between the two arms (see Table 13).
Table 13. Demographics and Baseline Characteristics in TOWER Study Characteristics BLINCYTO
(N = 271)Standard of Care (SOC) Chemotherapy
(N = 134)- *
- alloHSCT = allogeneic hematopoietic stem cell transplantation.
Age Median, years (min, max) 37 (18, 80) 37 (18, 78) < 35 years, n (%) 124 (46) 60 (45) ≥ 35 years, n (%) 147 (54) 74 (55) ≥ 65 years, n (%) 33 (12) 15 (11) ≥ 75 years, n (%) 10 (4) 2 (2) Males, n (%) 162 (60) 77 (58) Race, n (%) American Indian or Alaska Native 4 (2) 1 (1) Asian 19 (7) 9 (7) Black (or African American) 5 (2) 3 (2) Multiple 2 (1) 0 Native Hawaiian or Other Pacific Islander 1 (0) 1 (1) Other 12 (4) 8 (6) White 228 (84) 112 (84) Prior salvage therapy 171 (63) 70 (52) Prior alloHSCT* 94 (35) 46 (34) Eastern Cooperative Group Status - n (%) 0 96 (35) 52 (39) 1 134 (49) 61 (46) 2 41 (15) 20 (15) Unknown 0 1 (1) Refractory to salvage treatment - n (%) Yes 87 (32) 34 (25) No 182 (67) 99 (74) Unknown 2 (1) 1 (1) Maximum of central/local bone marrow blasts - n (%) ≤ 5% 0 0 > 5 to < 10% 9 (3) 7 (5) 10 to < 50% 60 (22) 23 (17) ≥ 50% 201 (74) 104 (78) Unknown 1 (0) 0 Of the 271 patients randomized to the BLINCYTO arm, 267 patients received BLINCYTO treatment. The median number of treatment cycles was two (range: 1 to 9 cycles); 267 (99%) received Cycles 1-2 (induction), 86 (32%) received Cycles 3-5 (consolidation), and 27 (10%) received Cycles 6-9 (continued therapy). Of the 134 patients on the SOC arm, 25 dropped out prior to start of study treatment, and 109 patients received a median of 1 treatment cycle (range: 1 to 4 cycles).
The determination of efficacy was based on overall survival (OS). The study demonstrated statistically significant improvement in OS for patients treated with BLINCYTO as compared to SOC chemotherapy.
See Figure 1 and Table 14 below for efficacy results from the TOWER Study.
Table 14. Efficacy Results in Patients ≥ 18 Years of Age with Philadelphia Chromosome-Negative Relapsed or Refractory B-cell Precursor ALL (TOWER Study) BLINCYTO
(N = 271)SOC Chemotherapy
(N = 134)- *
- Based on stratified Cox's model.
- †
- The p-value was derived using stratified log rank test.
- ‡
- CR (complete remission) was defined as ≤ 5% blasts in the bone marrow, no evidence of disease, and full recovery of peripheral blood counts (platelets > 100,000/microliter and absolute neutrophil counts [ANC] > 1,000/microliter).
- §
- CRh* (complete remission with partial hematologic recovery) was defined as ≤ 5% blasts in the bone marrow, no evidence of disease, and partial recovery of peripheral blood counts (platelets > 50,000/microliter and ANC > 500/microliter).
- ¶
- The p-value was derived using Cochran-Mantel-Haenszel test.
- #
- MRD (minimum residual disease) response was defined as MRD by PCR or flow cytometry < 1 × 10-4 (0.01%).
- Þ
- n1: number of patients who achieved MRD response and CR/CRh*; n2: number of patients who achieved CR/CRh* and had a postbaseline assessment.
Overall Survival Number of deaths (%) 164 (61) 87 (65) Median, months [95% CI] 7.7 [5.6, 9.6] 4.0 [2.9, 5.3] Hazard Ratio [95% CI]* 0.71 [0.55, 0.93] p-value† 0.012 Overall Response CR‡/CRh*§, n (%) [95% CI] 115 (42) [37, 49] 27 (20) [14, 28] Treatment difference [95% CI] 22 [13, 31] p-value¶ < 0.001 CR, n (%) [95% CI] 91 (34) [28, 40] 21 (16) [10, 23] Treatment difference [95% CI] 18 [10, 26] p-value¶ < 0.001 MRD Response# for CR/CRh* n1/n2 (%)Þ [95% CI] 73/115 (64) [54, 72] 14/27 (52) [32, 71] Study MT103-211
Study MT103-211 [NCT01466179] was an open-label, multicenter, single-arm study. Eligible patients were ≥ 18 years of age with Philadelphia chromosome-negative relapsed or refractory B-cell precursor ALL (relapsed with first remission duration of ≤ 12 months in first salvage or relapsed or refractory after first salvage therapy or relapsed within 12 months of alloHSCT, and had ≥ 10% blasts in bone marrow).
BLINCYTO was administered as a continuous intravenous infusion. The recommended dose for this study was determined to be 9 mcg/day on Days 1-7 and 28 mcg/day on Days 8-28 for Cycle 1, and 28 mcg/day on Days 1-28 for subsequent cycles. Dose adjustment was possible in case of adverse events. The treated population included 185 patients who received at least 1 infusion of BLINCYTO; the median number of treatment cycles was 2 (range: 1 to 5). Patients who responded to BLINCYTO but later relapsed had the option to be retreated with BLINCYTO. Among treated patients, the median age was 39 years (range: 18 to 79 years), 63 out of 185 (34.1%) had undergone HSCT prior to receiving BLINCYTO, and 32 out of 185 (17.3%) had received more than 2 prior salvage therapies.
Efficacy was based on the complete remission (CR) rate, duration of CR, and proportion of patients with an MRD-negative CR/CR with partial hematological recovery (CR/CRh*) within 2 cycles of treatment with BLINCYTO. Table 15 shows the efficacy results from this study. The HSCT rate among those who achieved CR/CRh* was 39% (30 out of 77).
Table 15. Efficacy Results in Patients ≥ 18 Years of Age with Philadelphia Chromosome-Negative Relapsed or Refractory B-cell Precursor ALL (Study MT103-211) N = 185 CR* CRh*† CR/CRh* - *
- CR (complete remission) was defined as ≤ 5% of blasts in the bone marrow, no evidence of disease, and full recovery of peripheral blood counts (platelets > 100,000/microliter and absolute neutrophil counts [ANC] > 1,000/microliter).
- †
- CRh* (complete remission with partial hematological recovery) was defined as ≤ 5% of blasts in the bone marrow, no evidence of disease, and partial recovery of peripheral blood counts (platelets > 50,000/microliter and ANC > 500/microliter).
- ‡
- MRD (minimal residual disease) response was defined as MRD by PCR < 1 × 10-4 (0.01%).
- §
- n1: number of patients who achieved MRD response and the respective remission status; n2: number of patients who achieved the respective remission status. Six CR/CRh* responders with missing MRD data were considered as MRD-nonresponders.
- ¶
- DOR (duration of response)/RFS (relapse-free survival) was defined as time since first response of CR or CRh* to relapse or death, whichever is earlier. Relapse was defined as hematological relapse (blasts in bone marrow greater than 5% following CR) or an extramedullary relapse.
n (%)
[95% CI]60 (32.4)
[25.7, 39.7]17 (9.2)
[5.4, 14.3]77 (41.6)
[34.4, 49.1]MRD response‡ n1/n2 (%)§
[95% CI]48/60 (80.0)
[67.7, 89.2]10/17 (58.8)
[32.9, 81.6]58/77 (75.3)
[64.2, 84.4]DOR/RFS¶ Median (months) (range) 6.7 (0.46 – 16.5) 5.0 (0.13 – 8.8) 5.9 (0.13 – 16.5) ALCANTARA Study
The efficacy of BLINCYTO for treatment of Philadelphia chromosome-positive B-cell precursor ALL was evaluated in an open-label, multicenter, single-arm study (ALCANTARA Study) [NCT02000427]. Eligible patients were ≥ 18 years of age with Philadelphia chromosome-positive B-cell precursor ALL, relapsed or refractory to at least 1 second generation or later tyrosine kinase inhibitor (TKI), or intolerant to second generation TKI, and intolerant or refractory to imatinib mesylate.
BLINCYTO was administered at 9 mcg/day on Days 1-7 and 28 mcg/day on Days 8-28 for Cycle 1, and 28 mcg/day on Days 1-28 for subsequent cycles. Dose adjustment was possible in case of adverse events.
The treated population included 45 patients who received at least one infusion of BLINCYTO; the median number of treatment cycles was 2 (range: 1 to 5). The demographics and baseline characteristics are shown in Table 16.
Table 16. Demographics and Baseline Characteristics in ALCANTARA Study Characteristics BLINCYTO
(N = 45)Age Median, years (min, max) 55 (23, 78) ≥ 65 years and < 75 years, n (%) 10 (22) ≥ 75 years, n (%) 2 (4) Males, n (%) 24 (53) Race, n (%) Asian 1 (2) Black (or African American) 3 (7) Other 2 (4) White 39 (87) Disease History Prior TKI treatment*, n (%) 1 7 (16) 2 21 (47) ≥ 3 17 (38) Prior salvage therapy 31 (62) Prior alloHSCT† 20 (44) Bone marrow blasts‡ ≥ 50% to < 75% 6 (13) ≥ 75% 28 (62) Efficacy was based on the complete remission (CR) rate, duration of CR, and proportion of patients with an MRD-negative CR/CR with partial hematological recovery (CR/CRh*) within 2 cycles of treatment with BLINCYTO. Table 17 shows the efficacy results from ALCANTARA Study. Five of the 16 responding (31%) patients underwent allogeneic HSCT in CR/CRh* induced with BLINCYTO. There were 10 patients with documented T315I mutation; four achieved CR within 2 cycles of treatment with BLINCYTO.
Table 17. Efficacy Results in Patients ≥ 18 Years of Age with Philadelphia Chromosome-Positive Relapsed or Refractory B-cell Precursor ALL (ALCANTARA Study) N = 45 CR* CRh*† CR/CRh* - *
- CR (complete remission) was defined as ≤ 5% of blasts in the bone marrow, no evidence of disease, and full recovery of peripheral blood counts (platelets > 100,000/microliter and absolute neutrophil counts [ANC] > 1,000/microliter).
- †
- CRh* (complete remission with partial hematological recovery) was defined as ≤ 5% of blasts in the bone marrow, no evidence of disease, and partial recovery of peripheral blood counts (platelets > 50,000/microliter and ANC > 500/microliter).
- ‡
- MRD (minimal residual disease) response was defined as MRD by PCR < 1 × 10-4 (0.01%).
- §
- n1: number of patients who achieved MRD response and the respective remission status; n2: number of patients who achieved the respective remission status. Six CR/CRh* responders with missing MRD data were considered as MRD-nonresponders.
- ¶
- DOR (duration of response)/RFS (relapse-free survival) was defined as time since first response of CR or CRh* to relapse or death, whichever is earlier. Relapse was defined as hematological relapse (blasts in bone marrow greater than 5% following CR) or an extramedullary relapse.
- #
- NE = not estimable
n (%)
[95% CI]14 (31)
[18, 47]2 (4)
[1, 15]16 (36)
[22, 51]MRD response‡ n1/n2 (%)§
[95% CI]12/14 (86)
[57, 98]2/2 (100)
[16, 100]14/16 (88)
[62, 98]DOR/RFS¶ Median (months) (range) 6.7 (3.6 – 12.0) NE# (3.7 – 9.0) 6.7 (3.6 – 12.0) Study MT103-205
Study MT103-205 [NCT01471782] was an open-label, multicenter, single-arm study in pediatric patients with relapsed or refractory B-cell precursor ALL (second or later bone marrow relapse, any marrow relapse after allogeneic HSCT, or refractory to other treatments, and had > 25% blasts in bone marrow). BLINCYTO was administered at 5 mcg/m2/day on Days 1-7 and 15 mcg/m2/day on Days 8-28 for Cycle 1, and 15 mcg/m2/day on Days 1-28 for subsequent cycles. Dose adjustment was possible in case of adverse events. Patients who responded to BLINCYTO but later relapsed had the option to be retreated with BLINCYTO.
Among the 70 treated patients, the median age was 8 years (range: 7 months to 17 years), 40 out of 70 (57.1%) had undergone allogeneic HSCT prior to receiving BLINCYTO, and 39 out of 70 (55.7%) had refractory disease. The median number of treatment cycles was 1 (range: 1 to 5).
Twenty-three out of 70 (32.9%) patients achieved CR/CRh* within the first 2 treatment cycles with 17 out of 23 (73.9%) occurring within Cycle 1 of treatment. See Table 18 for the efficacy results from the study. The HSCT rate among those who achieved CR/CRh* was 48% (11 out of 23).
Table 18. Efficacy Results in Patients < 18 Years of Age with Relapsed or Refractory B-cell Precursor ALL (Study MT103-205) N = 70 CR* CRh*† CR/CRh* - *
- CR (complete remission) was defined as ≤ 5% of blasts in the bone marrow, no evidence of circulating blasts or extra-medullary disease, and full recovery of peripheral blood counts (platelets > 100,000/microliter and absolute neutrophil counts [ANC] > 1,000/microliter).
- †
- CRh* (complete remission with partial hematological recovery) was defined as ≤ 5% of blasts in the bone marrow, no evidence of circulating blasts or extramedullary disease, and partial recovery of peripheral blood counts (platelets > 50,000/microliter and ANC > 500/microliter).
- ‡
- MRD (minimal residual disease) response was defined as MRD by PCR or flow cytometry < 1 × 10-4 (0.01%).
- §
- n1: number of patients who achieved MRD response and the respective remission status; n2: number of patients who achieved the respective remission status. One CR/CRh* responder with missing MRD data was considered as a MRD-nonresponder.
- ¶
- DOR (duration of response)/RFS (relapse-free survival) was defined as time since first response of CR or CRh* to relapse or death, whichever is earlier. Relapse was defined as hematological relapse (blasts in bone marrow greater than 5% following CR) or an extramedullary relapse.
n (%)
[95% CI]12 (17.1)
[9.2, 28.0]11 (15.7)
[8.1, 26.4]23 (32.9)
[22.1, 45.1]MRD response‡ n1/n2 (%)§
[95% CI]6/12 (50.0)
[21.1, 78.9]4/11 (36.4)
[10.9, 69.2]10/23 (43.5)
[23.2, 65.5]DOR/RFS¶ Median (months) (range) 6.0 (0.5 – 12.1) 3.5 (0.5 – 16.4) 6.0 (0.5 – 16.4) Close14.3 Philadelphia Chromosome-Negative B-cell Precursor ALL in the Consolidation Phase
Study E1910
The efficacy of BLINCYTO was evaluated in a randomized, controlled study in adult patients with newly diagnosed Philadelphia chromosome-negative B-cell precursor ALL (Study E1910) [NCT02003222]. Eligible patients in hematologic complete remission (CR) or CR with incomplete peripheral blood count recovery (CRi) following induction and intensification chemotherapy were randomized 1:1 to receive a consolidation regimen comprised of multiple cycles of BLINCYTO monotherapy in addition to multiple cycles of intensive chemotherapy (BLINCYTO arm) or to intensive chemotherapy alone (chemotherapy arm). Randomization was stratified by age (< 55 years versus ≥ 55 years), CD20 status, rituximab use, and intent to undergo allogeneic stem cell transplantation (HSCT).
The postremission treatment consisted of a BFM-like chemotherapy regimen adapted from the E2993/UKALLXII clinical trial. Patients randomized to the BLINCYTO arm were to receive 2 cycles of BLINCYTO followed by 3 cycles of consolidation chemotherapy, then a third cycle of BLINCYTO followed by the fourth cycle of chemotherapy and a fourth cycle of BLINCYTO (total 8 cycles). BLINCYTO was administered as a continuous intravenous infusion at 28 mcg/day on Days 1-28. Patients randomized to the chemotherapy arm of the study were to receive 4 cycles of chemotherapy alone (total 4 cycles). Patients on the BLINCYTO arm could go to HSCT after 1 - 2 cycles of BLINCYTO and up to 2 cycles of consolidation chemotherapy, and patients randomized to the chemotherapy arm could go to HSCT after intensification and up to 3 cycles of consolidation chemotherapy. All patients who completed consolidation but did not go to HSCT received maintenance therapy through 2 1/2 years from the start of intensification.
The demographics and baseline characteristics are provided in Table 19.
Table 19. Demographics and Baseline Characteristics in Study E1910 Characteristics Consolidation Consisting of BLINCYTO Cycles + Chemotherapy Cycles
(n = 112)Chemotherapy Cycles Alone
(n = 112)- *
- allogeneic SCT = allogeneic stem cell transplantation.
Age Median, years (min, max) 52 (31, 69) 50 (30, 70) Males, n (%) 55 (49) 56 (50) Race, n (%) American Indian or Alaska Native 2 (2) 1 (1) Asian 3 (3) 2 (2) Black (or African American) 9 (8) 4 (4) Native Hawaiian or Other Pacific Islander 1 (1) 0 White 87 (78) 89 (79) Not Reported 5 (4) 6 (5) Unknown 5 (4) 10 (9) Ethnicity, n (%) Hispanic or Latino 13 (12) 10 (9) Not Hispanic or Latino 95 (85) 95 (85) Not Reported 1 (1) 2 (2) Unknown 3 (3) 5 (4) Stratification Factors, n (%) Age < 55 years at randomization 65 (58) 65 (58) CD20 positive 45 (40) 46 (41) Rituximab use 33 (29) 36 (32) Planned allogeneic SCT* 36 (32) 35 (31) Efficacy was established on the basis of overall survival (OS). The results with a median follow-up of 3.6 years are shown in Figure 2 and Table 20.
Figure 2. Kaplan-Meier for Overall Survival in Study E1910 KM = Kaplan-Meier. CI = Confidence Interval. N = Number of patients in the analysis set.
Censor indicated by vertical bar.
Table 20. Overall Survival in Study E1910 BLINCYTO + Chemotherapy Chemotherapy CI = Confidence interval. Overall survival (OS) is calculated from time of randomization until death due to any cause. Number of patients 112 112 Overall Survival 3-year Kaplan-Meier estimate (%) [95% CI] 84.8 [76.3, 90.4] 69.0 [58.7, 77.2] Hazard ratio [95% CI]* 0.42 [0.24, 0.75] p-value† 0.003 In a later analysis with a median follow-up of 4.5 years, the 5-year OS was 82.4 % [95% CI (73.7, 88.4)] in the BLINCYTO + chemotherapy arm and 62.5 % [95% CI (52.0, 71.3)] in the chemotherapy arm. The hazard ratio was 0.44 [95% CI (0.25, 0.76)].
Study 20120215
The efficacy of BLINCYTO compared to consolidation chemotherapy was evaluated in a randomized, controlled, open-label, multicenter study (Study 20120215) [NCT02393859]. Eligible patients were 28 days to 18 years old and had high-risk, first-relapsed, Philadelphia chromosome-negative B-cell precursor ALL with < 25% blasts in the bone marrow after induction and 2 cycles of consolidation chemotherapy. Patients were randomized 1:1 to receive BLINCYTO or the IntReALLHR2010 HC3 intensive combination chemotherapy as the third cycle of consolidation. Patients in the BLINCYTO arm received one cycle of BLINCYTO as a continuous intravenous infusion at 15 mcg/m2/day over 4 weeks (maximum daily dose was not to exceed 28 mcg/day). Randomization was stratified by age, minimal residual disease status determined at the end of induction based on local assessment, and bone marrow status determined at the end of the second block of consolidation chemotherapy. Patients were to proceed to HSCT after this cycle of consolidation.
There were 54 patients randomized to the BLINCYTO arm and 57 to the chemotherapy arm. The demographics and baseline characteristics are shown in Table 21.
Table 21. Demographics and Baseline Characteristics in Study 20120215 Characteristics Consolidation Cycle 3 BLINCYTO
(N = 54)Chemotherapy
(N = 57)N = number of patients in the analysis set; n = number of patients with observed data; MRD = minimal residual disease; PCR = polymerase chain reaction. Age, n (%) Median, (range) 6 (1, 17) 5 (1, 17) < 1 year 0 0 1 to 9 years 39 (72) 41 (72) ≥ 10 to 18 years 15 (28) 16 (28) Males, n (%) 30 (56) 23 (40) Race, n (%) American Indian or Alaska Native 0 0 Asian 1 (2) 3 (5) Black (or African American) 0 3 (5) Native Hawaiian or Other Pacific Islander 0 0 Other 3 (6) 5 (9) White 50 (93) 46 (81) Cytomorphology at randomization, n (%) Blasts < 5% 54 (100) 54 (95) Blasts ≥ 5% and < 25% 0 2 (4) Blasts ≥ 25% blasts 0 0 Not evaluable 0 1 (2) MRD PCR value at randomization, n (%) ≥ 10-3 11 (20) 16 (28) < 10-3 and ≥ 10-4 15 (28) 6 (11) < 10-4 20 (37) 23 (40) Unknown 8 (15) 12 (21) Time from first diagnosis to relapse (month), n (%) < 18 months 19 (35) 22 (39) ≥ 18 months and ≤ 30 months 32 (59) 31 (54) > 30 months 3 (6) 4 (7) Efficacy was established on the basis of overall survival (OS) and relapse-free survival (RFS). See Table 22, Figure 3, and Figure 4 for results of OS and RFS from Study 20120215.
Table 22. Efficacy Results in Pediatric Patients with High-Risk First Relapsed B-cell Precursor ALL (Study 20120215) Consolidation Cycle 3 BLINCYTO
(N = 54)Chemotherapy
(N = 57)NE = Not estimable. CI = Confidence interval. Overall Survival Number of deaths (%) 11 (20.4) 28 (49.1) 5-year KM estimate (%) [95% CI]* 78.4 [64.2, 87.4] 41.4 [26.3, 55.9] Hazard Ratio [95% CI]† 0.35 [0.17, 0.70] Relapse-free Survival Events, n (%) 20 (37.0) 37 (64.9) 5-year KM estimate (%) [95% CI]* 61.1 [46.3, 72.9] 27.6 [16.2, 40.3] Hazard Ratio [95% CI]† 0.38 [0.22, 0.66] The median follow-up time for OS was 55.2 months for the overall population. Figure 3 presents a Kaplan-Meier plot comparing OS between treatment arms for the overall population.
Figure 3. Kaplan-Meier for Overall Survival (Study 20120215) KM = Kaplan-Meier. CI = Confidence Interval. N = Number of patients in the analysis set.
Censor indicated by vertical bar.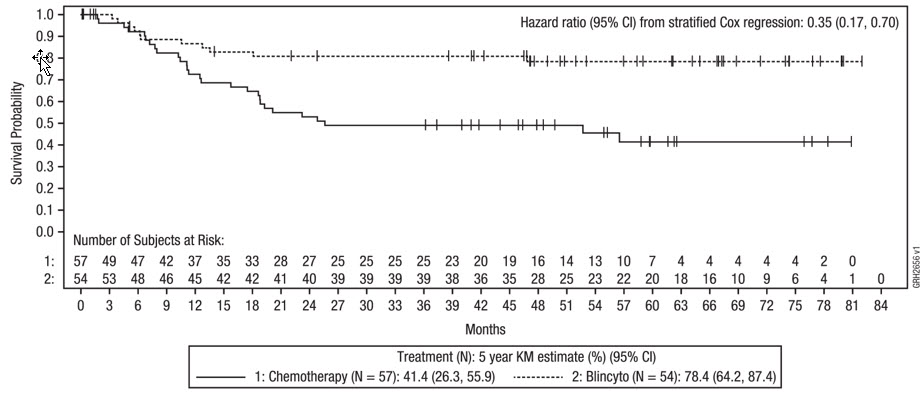
Figure 4. Kaplan-Meier for Relapse-free Survival (Study 20120215) KM = Kaplan-Meier. CI = Confidence Interval. N = Number of patients in the analysis set.
Censor indicated by vertical bar.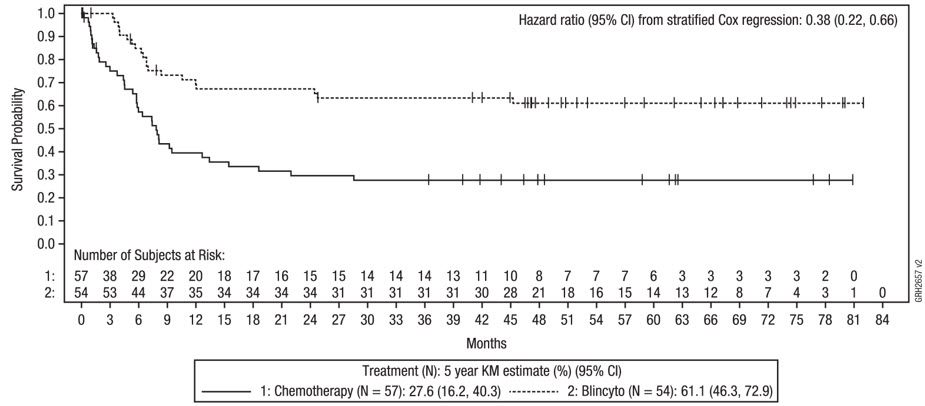
-
16 HOW SUPPLIED/STORAGE AND HANDLINGEach BLINCYTO package (NDC 55513-160-01) contains: One BLINCYTO (blinatumomab) for injection 35 mcg single-dose vial containing a sterile, preservative-free, white to off-white lyophilized powder ...
Each BLINCYTO package (NDC 55513-160-01) contains:
- One BLINCYTO (blinatumomab) for injection 35 mcg single-dose vial containing a sterile, preservative-free, white to off-white lyophilized powder and
- One IV Solution Stabilizer 10 mL single-dose glass vial containing a sterile, preservative-free, colorless to slightly yellow, clear solution.
CloseStore BLINCYTO and IV Solution Stabilizer vials in the original package refrigerated at 2°C to 8°C (36°F to 46°F) and protect from light until time of use. Do not freeze.
BLINCYTO and IV Solution Stabilizer vials may be stored for a maximum of 8 hours at room temperature [23°C to 27°C (73°F to 81°F)] in the original carton to protect from light.
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Cytokine Release Syndrome (CRS) Advise patients of the risk of CRS and infusion reactions, and to contact ...
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Cytokine Release Syndrome (CRS)
Advise patients of the risk of CRS and infusion reactions, and to contact their healthcare professional for signs and symptoms associated with CRS or infusion reactions (pyrexia, fatigue, nausea, vomiting, chills, hypotension, rash, and wheezing) [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)].
Neurological Toxicities, including Immune Effector Cell-Associated Neurotoxicity Syndrome
Advise patients of the risk of neurological toxicities, including ICANS, and to contact their healthcare professional for signs and symptoms associated with this event (including convulsions, speech disorders, and confusion) [see Warnings and Precautions (5.2) and Adverse Reactions (6.1)].
Infections
Advise patients of the risk of infections, and to contact their healthcare professional for signs or symptoms of infection [see Warnings and Precautions (5.3) and Adverse Reactions (6.1)].
Inform patients of the importance of keeping the skin clean around the intravenous catheter to reduce the risk of infection.
Pancreatitis
Advise patients of the risk of pancreatitis and to contact their healthcare provider for signs or symptoms of pancreatitis, which include severe and persistent stomach pain, with or without nausea and vomiting [see Warnings and Precautions (5.8) and Adverse Reactions (6.2)].
Driving and Engaging in Hazardous Occupations
Advise patients to refrain from driving and engaging in hazardous occupations or activities such as operating heavy or potentially dangerous machinery while BLINCYTO is being administered. Patients should be advised that they may experience neurological events, including seizures and ICANS [see Warnings and Precautions (5.6)].
Infusion Pump Errors
Inform patients they should not adjust the setting on the infusion pump. Any changes to pump function may result in dosing errors. If there is a problem with the infusion pump or the pump alarms, patients should contact their doctor or nurse immediately.
Embryo-Fetal Toxicity
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to inform their healthcare provider if they are pregnant or become pregnant [see Warnings and Precautions (5.13) and Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with BLINCYTO and for 48 hours after the last dose [see Warnings and Precautions (5.13) and Use in Specific Populations (8.3)].
CloseLactation
Advise women not to breastfeed during treatment with BLINCYTO and for 48 hours after the last dose [see Use in Specific Populations (8.2)].
-
SPL UNCLASSIFIED SECTIONManufactured by: Amgen Inc. One Amgen Center Drive - Thousand Oaks, California 91320-1799 - U.S. License No. 1080 - BLINCYTO® (blinatumomab) Patent: http://pat.amgen.com/blincyto/ © 2014-2024 Amgen ...
Manufactured by:
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, California 91320-1799
U.S. License No. 1080BLINCYTO® (blinatumomab)
Patent: http://pat.amgen.com/blincyto/
© 2014-2024 Amgen Inc. All rights reserved.
Close
V17 -
MEDICATION GUIDEThis Medication Guide has been approved by the U.S. Food and Drug Administration.Revised: 12/2024 Medication Guide - BLINCYTO® (blin sye toe) (blinatumomab) for injection - What is ...Close
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 12/2024 Medication Guide
BLINCYTO® (blin sye toe)
(blinatumomab)
for injectionWhat is the most important information I should know about BLINCYTO?
Call your healthcare provider or get emergency medical help right away if you get any of the symptoms listed below.
BLINCYTO may cause serious side effects that can be severe, life-threatening, or lead to death, including:- Cytokine Release Syndrome (CRS) and Infusion Reactions. Symptoms of CRS and infusion reactions may include:
- fever
- tiredness or weakness
- dizziness
- headache
- low blood pressure
- nausea
- vomiting
- chills
- face swelling
- wheezing or trouble breathing
- skin rash
- Neurologic problems. Symptoms of neurologic problems may include:
- seizures
- difficulty in speaking or slurred speech
- loss of consciousness
- trouble sleeping
- confusion and disorientation
- loss of balance
- headache
- difficulty with facial movements, hearing, vision, or swallowing
- tremors
People with Down Syndrome may have a higher risk of seizures with BLINCYTO treatment. Your healthcare provider will check for these problems during treatment with BLINCYTO. Your healthcare provider may temporarily stop or completely stop your treatment with BLINCYTO, if you have severe side effects.
See “What are the possible side effects of BLINCYTO?” below for other side effects of BLINCYTO.What is BLINCYTO?
BLINCYTO is a prescription medicine used to treat adults and children 1 month and older with:
- B-cell precursor acute lymphoblastic leukemia (ALL) in remission when only a small number of cancer cells remain in the body (minimal residual disease)
- B-cell precursor ALL that has come back or did not respond to previous treatments
- Philadelphia-chromosome negative B-cell precursor ALL in the consolidation phase of chemotherapy treatment with multiple phases
It is not known if BLINCYTO is safe and effective in children less than 1 month of age.Who should not receive BLINCYTO?
Do not receive BLINCYTO if you are allergic to blinatumomab or to any of the ingredients of BLINCYTO. See the end of this Medication Guide for a complete list of ingredients in BLINCYTO.Before receiving BLINCYTO, tell your healthcare provider about all of your medical conditions, including if you or your child:
- have a history of neurological problems, such as seizures, confusion, trouble speaking or loss of balance
- have Down Syndrome
- have an infection
- have ever had an infusion reaction after receiving BLINCYTO or other medications
- have a history of radiation treatment to the brain, or chemotherapy treatment
- are scheduled to receive a vaccine. You should not receive a “live vaccine” for at least 2 weeks before you start treatment with BLINCYTO, during treatment, and until your immune system recovers after you receive your last cycle of BLINCYTO. If you are not sure about the type of vaccine, ask your healthcare provider.
- are pregnant or plan to become pregnant. BLINCYTO may harm your unborn baby. Tell your healthcare provider if you become pregnant during treatment with BLINCYTO.
- If you are able to become pregnant, your healthcare provider should do a pregnancy test before you start treatment with BLINCYTO.
- Females who are able to become pregnant should use an effective form of birth control (contraception) during treatment with BLINCYTO, and for 48 hours after your last dose of BLINCYTO.
- are breastfeeding or plan to breastfeed. It is not known if BLINCYTO passes into your breast milk. You should not breastfeed during treatment with BLINCYTO and for 48 hours after your last dose.
How will I receive BLINCYTO?
- BLINCYTO will be given to you by intravenous (IV) infusion into your vein by an infusion pump.
- Your healthcare provider will decide the number of treatment cycles of BLINCYTO.
- You will receive BLINCYTO by continuous IV infusion for 4 weeks (28 days), followed by a 2-week (14 days) break during which you will not receive BLINCYTO. This is 1 treatment cycle (42 days).
- Your healthcare provider may prescribe continued therapy.
- You will receive BLINCYTO by continuous IV infusion for 4 weeks (28 days), followed by an 8-week (56 days) break during which you will not receive BLINCYTO. This is 1 treatment cycle (84 days).
- Your healthcare provider may give you BLINCYTO in a hospital or clinic for the first 3 or 9 days of the first treatment cycle and for the first 2 days of the second cycle to check you for side effects. If you receive additional treatment cycles of BLINCYTO or if your treatment is stopped for a period of time and restarted, you may also be treated in a hospital or clinic.
- Your healthcare provider may change your dose of BLINCYTO, delay, or completely stop treatment with BLINCYTO if you have certain side effects.
- Your healthcare provider will do blood tests during treatment with BLINCYTO to check you for side effects.
- Before you receive BLINCYTO, you will be given a corticosteroid medicine to help reduce infusion reactions.
- Before and during treatment with BLINCYTO, you may be given chemotherapy as an injection into the space that surrounds the spinal cord and the brain (intrathecal injection) to help prevent central nervous system relapse of ALL.
- It is very important to keep the area around the IV catheter clean to reduce the risk of getting an infection. Your healthcare provider will show you how to care for your catheter site.
- Do not change the settings on your infusion pump, even if there is a problem with your pump or your pump alarm sounds. Any changes to your infusion pump settings may cause a dose that is too high or too low to be given.
What should I avoid while receiving BLINCYTO?
Do not drive, operate heavy machinery, or do other dangerous activities while you are receiving BLINCYTO because BLINCYTO can cause neurological symptoms, such as dizziness, seizures, and confusion.What are the possible side effects of BLINCYTO?
BLINCYTO may cause serious side effects, including:
See “What is the most important information I should know about BLINCYTO?”- Infections. BLINCYTO may cause life-threatening infections that may lead to death. Tell your healthcare provider right away if you develop any signs or symptoms of an infection.
- Tumor Lysis Syndrome (TLS). TLS is caused by the fast breakdown of cancer cells. TLS can be life-threatening and may lead to death. Tell your healthcare provider right away if you have any symptoms of TLS during treatment with BLINCYTO, including:
- nausea and vomiting
- confusion
- shortness of breath
- irregular heartbeat
- dark or cloudy urine
- reduced amount of urine
- unusual tiredness
- muscle cramps
-
Low white blood cell counts (neutropenia). Neutropenia is common with BLINCYTO treatment and may sometimes be life-threatening. Low white blood cell counts can increase your risk of infection. Your healthcare provider will do blood tests to check your white blood cell count during treatment with BLINCYTO. Tell your healthcare provider right away if you get a fever.
-
Abnormal liver blood tests. Your healthcare provider will do blood tests to check your liver before you start BLINCYTO and during treatment with BLINCYTO.
- Inflammation of the pancreas (pancreatitis). Pancreatitis may happen in people treated with BLINCYTO and corticosteroids. It may be severe and lead to death. Tell your healthcare provider right away if you have severe stomach-area pain that does not go away. The pain may happen with or without nausea and vomiting.
- fever
- reactions related to infusion of the medicine such as face swelling, low blood pressure, and high blood pressure (infusion-related reactions)
- headache
- infection
- muscle, joint and bone pain
- low white blood cell count (neutropenia)
- nausea
- low red blood cell count (anemia)
- low platelet count (thrombocytopenia)
- diarrhea
These are not all the possible side effects of BLINCYTO.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store BLINCYTO?
Intravenous (IV) bags containing BLINCYTO for infusion will arrive in a special package.
- Do not open the package.
- Do not freeze the package.
- The package containing BLINCYTO will be opened by your healthcare provider and stored in the refrigerator at 36°F to 46°F (2°C to 8°C).
- Do not throw away (dispose of) any BLINCYTO in your household trash. Talk with your healthcare provider about disposal of BLINCYTO and used supplies.
General information about safe and effective use of BLINCYTO.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use BLINCYTO for a condition for which it was not prescribed. Do not give BLINCYTO to other people even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about BLINCYTO that is written for health professionals.What are the ingredients in BLINCYTO?
Active ingredient: blinatumomab
Inactive ingredients: citric acid monohydrate, lysine hydrochloride, polysorbate 80, trehalose dihydrate, sodium hydroxide and preservative-free sterile water for injection.
Inactive ingredients of IV Solution Stabilizer: citric acid monohydrate, lysine hydrochloride, polysorbate 80, sodium hydroxide and water for injection.
Manufactured by: Amgen Inc., One Amgen Center Drive, Thousand Oaks, CA 91320-1799
U.S. License No. 1080 © 2014-2024 Amgen Inc. All rights reserved. v12
For more information, go to www.blincyto.com or call Amgen at 1-800-772-6436. -
SPL UNCLASSIFIED SECTION24-Hour or 48-Hour Infusion - INSTRUCTIONS FOR USE: 24-HOUR OR 48-HOUR INFUSION - The preparation steps differ based on the infusion duration. Follow the steps specific to the infusion ...
24-Hour or 48-Hour Infusion INSTRUCTIONS FOR USE: 24-HOUR OR 48-HOUR INFUSION
The preparation steps differ based on the infusion duration.
Follow the steps specific to the infusion duration you are preparing.
It is very important that the instructions for preparation (including admixing) and administration provided in this section are strictly followed to minimize medication errors (including underdose and overdose) [see Dosage and Administration (2.5), Warnings and Precautions (5.10)].Aseptic Preparation
Strictly observe aseptic technique when preparing the solution for infusion since BLINCYTO vials do not contain antimicrobial preservatives. To prevent accidental contamination, prepare BLINCYTO according to aseptic standards, including but not limited to:- Prepare BLINCYTO in a USP <797> compliant facility.
- Prepare BLINCYTO in an ISO Class 5 laminar flow hood or better.
- Ensure that the admixing area has appropriate environmental specifications, confirmed by periodic monitoring.
- Ensure that personnel are appropriately trained in aseptic manipulations and admixing of oncology drugs.
- Ensure that personnel wear appropriate protective clothing and gloves.
- Ensure that gloves and surfaces are disinfected.
- Preservative-Free Sterile Water for Injection, USP.
- Preservative-Free 0.9% Sodium Chloride Injection, USP.
- Sterile, non-pyrogenic, low protein-binding, 0.2 micron in-line filter.
- Infusion bags/pump cassettes and intravenous tubing sets: Use either polyolefin, DEHP-free PVC, or ethyl vinyl acetate (EVA).
- -
- BLINCYTO is incompatible with diethylhexylphthalate (DEHP) due to the possibility of particle formation, leading to a cloudy solution.
- BLINCYTO package(s), each BLINCYTO package contains:
- -
- One BLINCYTO for injection 35 mcg single-dose vial containing a sterile, preservative-free, white to off-white lyophilized powder.
- -
- More than one vial of BLINCYTO may be needed to prepare the recommended dose.
- -
- One IV Solution Stabilizer 10 mL single-dose glass vial containing a sterile, preservative-free, colorless to slightly yellow, clear solution.
- -
- Do not use IV Solution Stabilizer for reconstitution of BLINCYTO.
- -
- IV Solution Stabilizer is used to coat the intravenous bag prior to addition of reconstituted BLINCYTO to prevent adhesion of BLINCYTO to intravenous bags and intravenous tubing.
Preparation of BLINCYTO: Reconstitution - 1.
-
Determine the number of BLINCYTO vials needed for a dose and infusion duration.
- Refer to Table 1 (patients weighing 45 kg or more) or Table 2 (patients weighing less than 45 kg).
- a.
- Reconstitute each BLINCYTO vial with 3 mL of preservative-free Sterile Water for Injection, USP by directing the water along the walls of the BLINCYTO vial and not directly on the lyophilized powder. The resulting concentration per BLINCYTO vial is 12.5 mcg/mL.
- Do not reconstitute BLINCYTO vials with the IV Solution Stabilizer (IVSS).





3 mL of Preservative-Free Sterile Water for Injection, USP Lyophilized BLINCYTO Reconstituted BLINCYTO 
Important: Do not reconstitute BLINCYTO vials with IV Solution Stabilizer (IVSS). - b.
- Gently swirl contents to avoid excess foaming.
- Do not shake.
- c.
-
Visually inspect the reconstituted solution for particulate matter and discoloration during reconstitution and prior to preparing the intravenous bag. The resulting solution should be clear to slightly opalescent, colorless to slightly yellow.
- Do not use if solution is cloudy or has precipitated.
- 2.
- Aseptically add 270 mL of preservative-free 0.9% Sodium Chloride Injection, USP to the empty intravenous bag.

270 mL of Preservative-Free 0.9% NaCl Injection, USP 

Empty IV Bag Material, use either: - Polyolefin,
- DEHP-free PVC, or
- EVA IV Bag
- 3.
- Aseptically transfer 5.5 mL of IV Solution Stabilizer (IVSS) to the intravenous bag containing preservative-free 0.9% Sodium Chloride Injection, USP.
- Gently mix the contents of the bag to avoid foaming.

IV Solution Stabilizer - For 24-hour infusion, transfer 5.5 mL IV Solution Stabilizer.
- For 48-hour infusion, transfer 5.5 mL IV Solution Stabilizer.
- Discard the vial containing the unused IV Solution Stabilizer.
- 4.
- Aseptically transfer the required volume of reconstituted BLINCYTO solution into the intravenous bag containing preservative-free 0.9% Sodium Chloride Injection, USP and IV Solution Stabilizer.
- Gently mix the contents of the bag to avoid foaming.

Reconstituted BLINCYTO - Refer to Table 1 for patients weighing 45 kg or more for the specific volume of reconstituted BLINCYTO.
- Refer to Table 2 for patients weighing less than 45 kg (dose based on BSA) for the specific volume of reconstituted BLINCYTO.
- Discard the vial containing unused BLINCYTO.
- 5.
- Remove air from the intravenous bag. This is particularly important for use with an ambulatory infusion pump.

- 6.
- Under aseptic conditions, attach the intravenous tubing to the intravenous bag with the sterile 0.2 micron in-line filter.
- Ensure that the intravenous tubing is compatible with the infusion pump.
- Use either polyolefin, DEHP-free PVC or EVA intravenous tubing sets.
- 7.
-
Prime the intravenous tubing only with the solution in the bag containing the FINAL prepared BLINCYTO solution for infusion.

- 8.
- Store refrigerated at 2°C to 8°C (36°F to 46°F) if not used immediately [see Dosage and Administration (2.6)].
Table 1. For 24-Hour and 48-Hour Infusion: Patients Weighing 45 kg or More Infusion Duration Dose Reconstituted BLINCYTO Volume Vials 24 hours 9 mcg/day 0.83 mL 1 28 mcg/day 2.6 mL 1 48 hours 9 mcg/day 1.7 mL 1 28 mcg/day 5.2 mL 2 Table 2. For 24-Hour and 48-Hour Infusion: Patients Weighing Less Than 45 kg Infusion Duration Dose BSA (m2) Reconstituted BLINCYTO Volume Vials 24 hours 5 mcg/m2/day 1.5 – 1.59 0.7 mL 1 1.4 – 1.49 0.66 mL 1 1.3 – 1.39 0.61 mL 1 1.2 – 1.29 0.56 mL 1 1.1 – 1.19 0.52 mL 1 1 – 1.09 0.47 mL 1 0.9 – 0.99 0.43 mL 1 0.8 – 0.89 0.38 mL 1 0.7 – 0.79 0.33 mL 1 0.6 – 0.69 0.29 mL 1 0.5 – 0.59 0.24 mL 1 0.4 – 0.49 0.2 mL 1 0.35 – 0.39 0.17 mL 1 0.3 – 0.34 0.15 mL 1 0.25 – 0.29 0.12 mL 1 0.2 – 0.24 0.1 mL 1 24 hours 15 mcg/m2/day 1.5 – 1.59 2.1 mL 1 1.4 – 1.49 2 mL 1 1.3 – 1.39 1.8 mL 1 1.2 – 1.29 1.7 mL 1 1.1 – 1.19 1.6 mL 1 1 – 1.09 1.4 mL 1 0.9 – 0.99 1.3 mL 1 0.8 – 0.89 1.1 mL 1 0.7 – 0.79 1 mL 1 0.6 – 0.69 0.86 mL 1 0.5 – 0.59 0.72 mL 1 0.4 – 0.49 0.59 mL 1 0.35 – 0.39 0.51 mL 1 0.3 – 0.34 0.44 mL 1 0.25 – 0.29 0.37 mL 1 0.2 – 0.24 0.3 mL 1 48 hours 5 mcg/m2/day 1.5 – 1.59 1.4 mL 1 1.4 – 1.49 1.3 mL 1 1.3 – 1.39 1.2 mL 1 1.2 – 1.29 1.1 mL 1 1.1 – 1.19 1 mL 1 1 – 1.09 0.94 mL 1 0.9 – 0.99 0.85 mL 1 0.8 – 0.89 0.76 mL 1 0.7 – 0.79 0.67 mL 1 0.6 – 0.69 0.57 mL 1 0.5 – 0.59 0.48 mL 1 0.4 – 0.49 0.39 mL 1 0.35 – 0.39 0.34 mL 1 0.3 – 0.34 0.29 mL 1 0.25 – 0.29 0.25 mL 1 0.2 – 0.24 0.2 mL 1 48 hours 15 mcg/m2/day 1.5 – 1.59 4.2 mL 2 1.4 – 1.49 3.9 mL 2 1.3 – 1.39 3.7 mL 2 1.2 – 1.29 3.4 mL 2 1.1 – 1.19 3.1 mL 2 1 – 1.09 2.8 mL 1 0.9 – 0.99 2.6 mL 1 0.8 – 0.89 2.3 mL 1 0.7 – 0.79 2 mL 1 0.6 – 0.69 1.7 mL 1 0.5 – 0.59 1.4 mL 1 0.4 – 0.49 1.2 mL 1 0.35 – 0.39 1 mL 1 0.3 – 0.34 0.88 mL 1 0.25 – 0.29 0.75 mL 1 0.2 – 0.24 0.61 mL 1 Administration of BLINCYTO: 24-Hour or 48-Hour Intravenous Bag - Administer BLINCYTO as a continuous intravenous infusion at a constant flow rate using an infusion pump. The pump should be programmable, lockable, non-elastomeric, and have an alarm.
- The starting volume (270 mL) is more than the volume administered to the patient (240 mL) to account for the priming of the intravenous tubing and to ensure that the patient will receive the full dose of BLINCYTO.
- Ensure that the intravenous tubing is primed only with the solution in the bag containing the FINAL prepared BLINCYTO solution for infusion.
- Administer the FINAL prepared BLINCYTO infusion solution using intravenous tubing that contains a sterile, non-pyrogenic, low protein-binding, 0.2 micron in-line filter for 24-hour or 48-hour bags.
- -
- For 72-hour, 96-hour, or 7-day bag administration information [see "Administration of BLINCYTO: 72-Hour, 96-Hour, or 7-Day Intravenous Bag"].
- Infuse the FINAL prepared BLINCYTO infusion solution according to the instructions on the pharmacy label on the prepared bag at one of the following constant infusion rates:
- -
- Infusion rate of 10 mL/hour for a duration of 24 hours, OR
- -
- Infusion rate of 5 mL/hour for a duration of 48 hours.
- Important Note: Do not flush the BLINCYTO infusion line, especially when changing infusion bags. Flushing when changing bags or at completion of infusion can result in excess dosage and complications thereof. When administering via a multi-lumen venous catheter, infuse BLINCYTO through a dedicated lumen. Before flushing the catheter system, residual amounts of BLINCYTO must be aspirated from the catheter system to avoid bolus administration.
- At the end of the infusion, any remaining solution in the intravenous bag and intravenous tubing should be discarded in accordance with local requirements.
72-Hour, 96-Hour, or 7-Day Infusion (Preservative) INSTRUCTIONS FOR USE: 72-HOUR OR 96-HOUR, OR 7-DAY INFUSION
The preparation steps differ based on the infusion duration.
Follow the steps specific to the infusion duration you are preparing.
It is very important that the instructions for preparation (including admixing) and administration provided in this section are strictly followed to minimize medication errors (including underdose and overdose) [see Dosage and Administration (2.5), Warnings and Precautions (5.10)].
The administration of BLINCYTO as a 72-hour, 96-hour, and 7-day infusion is not recommended for patients weighing less than 5.4 kg [see Warnings and Precautions (5.12)].Aseptic Preparation
Strictly observe aseptic technique when preparing the solution for infusion since BLINCYTO vials do not contain antimicrobial preservatives. To prevent accidental contamination, prepare BLINCYTO according to aseptic standards, including but not limited to:- Prepare BLINCYTO in a USP <797> compliant facility.
- Prepare BLINCYTO in an ISO Class 5 laminar flow hood or better.
- Ensure that the admixing area has appropriate environmental specifications, confirmed by periodic monitoring.
- Ensure that personnel are appropriately trained in aseptic manipulations and admixing of oncology drugs.
- Ensure that personnel wear appropriate protective clothing and gloves.
- Ensure that gloves and surfaces are disinfected.
- Preservative-Free Sterile Water for Injection, USP.
- Preservative-Free 0.9% Sodium Chloride Injection, USP.
- Bacteriostatic 0.9% Sodium Chloride Injection, USP.
- Infusion bags/pump cassettes and intravenous tubing sets: Use either polyolefin, DEHP-free PVC, or ethyl vinyl acetate (EVA).
- -
- BLINCYTO is incompatible with diethylhexylphthalate (DEHP) due to the possibility of particle formation, leading to a cloudy solution.
- BLINCYTO package(s), each BLINCYTO package contains:
- -
- One BLINCYTO for injection 35 mcg single-dose vial containing a sterile, preservative-free, white to off-white lyophilized powder.
- -
- More than one vial of BLINCYTO may be needed to prepare the recommended dose.
- -
- One IV Solution Stabilizer 10 mL single-dose glass vial containing a sterile, preservative-free, colorless to slightly yellow, clear solution.
- -
- Do not use IV Solution Stabilizer for reconstitution of BLINCYTO.
- -
- IV Solution Stabilizer is used to coat the intravenous bag prior to addition of reconstituted BLINCYTO to prevent adhesion of BLINCYTO to intravenous bags and intravenous tubing.
Preparation of BLINCYTO: Reconstitution - 1.
-
Determine the number of BLINCYTO vials needed for a dose and infusion duration.
- Refer to Table 3 (patients weighing 45 kg or more) or Table 4 (patients weighing between 5.4 kg and less than 45 kg).
- a.
- Reconstitute each BLINCYTO vial with 3 mL of preservative-free Sterile Water for Injection, USP by directing the water along the walls of the BLINCYTO vial and not directly on the lyophilized powder. The resulting concentration per BLINCYTO vial is 12.5 mcg/mL.
- Do not reconstitute BLINCYTO vials with the IV Solution Stabilizer (IVSS).





3 mL of Preservative-Free Sterile Water for Injection, USP Lyophilized BLINCYTO Reconstituted BLINCYTO 
Important: Do not reconstitute BLINCYTO vials with IV Solution Stabilizer (IVSS). - b.
- Gently swirl contents to avoid excess foaming.
- Do not shake.
- c.
-
Visually inspect the reconstituted solution for particulate matter and discoloration during reconstitution and prior to preparing the intravenous bag. The resulting solution should be clear to slightly opalescent, colorless to slightly yellow.
- Do not use if solution is cloudy or has precipitated.
- 2.
- Aseptically add the required volume of Bacteriostatic 0.9% Sodium Chloride Injection, USP to the empty intravenous bag.
- For 72-hour infusion, add 45 mL Bacteriostatic 0.9% Sodium Chloride Injection.
- For 96-hour infusion, add 56 mL Bacteriostatic 0.9% Sodium Chloride Injection.
- For 7-day infusion, add 90 mL Bacteriostatic 0.9% Sodium Chloride Injection.

Bacteriostatic 0.9% NaCl Injection, USP 

Empty IV Bag Material, use either: - Polyolefin,
- DEHP-free PVC, or
- EVA IV Bag
- 3.
- Aseptically transfer the required volume of IV Solution Stabilizer (IVSS) to the intravenous bag containing Bacteriostatic 0.9% Sodium Chloride Injection, USP.
- Gently mix the contents of the bag to avoid foaming.

IV Solution Stabilizer - For 72-hour infusion, transfer 3.2 mL IV Solution Stabilizer.
- For 96-hour infusion, transfer 4 mL IV Solution Stabilizer.
- For 7-day infusion, transfer 2.2 mL IV Solution Stabilizer.
- Discard the vial containing the unused IV Solution Stabilizer.
- 4.
- Aseptically transfer the required volume of reconstituted BLINCYTO solution into the intravenous bag containing Bacteriostatic 0.9% Sodium Chloride Injection, USP and IV Solution Stabilizer.
- Gently mix the contents of the bag to avoid foaming.

Reconstituted BLINCYTO - Refer to Table 3 for patients weighing 45 kg or more for the specific volume of reconstituted BLINCYTO.
- Refer to Table 4 for patients weighing between 5.4 kg and less than 45 kg (dose based on BSA) for the specific volume of reconstituted BLINCYTO.
- Discard the vial containing unused BLINCYTO.
- 5.
- Aseptically add the needed volume of preservative-free 0.9% Sodium Chloride Injection, USP to the intravenous bag to obtain the final volumes within Table 3 and Table 4.
- Gently mix the contents of the bag to avoid foaming.

Preservative-Free 0.9% NaCl Injection, USP - Refer to Table 3 for patients weighing 45 kg or more for the specific volume of preservative-free 0.9% Sodium Chloride Injection, USP.
- Refer to Table 4 for patients weighing between 5.4 kg and less than 45 kg (dose based on BSA) for the specific volume of preservative-free 0.9% Sodium Chloride Injection, USP.
- 6.
- Remove air from the intravenous bag. This is particularly important for use with an ambulatory infusion pump.

- 7.
- Under aseptic conditions, attach the intravenous tubing to the intravenous bag. Do not use an in-line filter for 72-hour, 96-hour, or 7-day bags.
- Ensure that the intravenous tubing is compatible with the infusion pump.
- Use either polyolefin, DEHP-free PVC or EVA intravenous tubing sets.

Important: Do not use an in-line filter for 72-hour, 96-hour, or 7-day bags. - 8.
-
Prime the intravenous tubing only with the solution in the bag containing the FINAL prepared BLINCYTO solution for infusion.

- 9.
- Store refrigerated at 2°C to 8°C (36°F to 46°F) if not used immediately [see Dosage and Administration (2.6)].
Table 3. For 72-Hour, 96-Hour, and 7-Day Infusion: Patients Weighing 45 kg or More Infusion Duration Dose Reconstituted BLINCYTO Volume of Preservative-Free 0.9% Sodium Chloride Injection, USP needed to q.s. to Final Volume Final Volume of IV Bag Volume Vials 72 hours 28 mcg/day 8.4 mL 3 105 mL 162 mL 96 hours 28 mcg/day 10.4 mL 4 130 mL 200 mL 7 days 28 mcg/day 16.8 mL 6 1 mL 110 mL Table 4. For 72-Hour, 96-Hour, and 7-Day Infusion: Patients Weighing Between 5.4 kg and Less Than 45 kg Infusion Duration Dose BSA (m2) Reconstituted BLINCYTO Volume of Preservative-Free 0.9% Sodium Chloride Injection, USP needed to q.s. to Final Volume Final Volume of IV Bag Volume Vials 72 hours 15 mcg/m2/day 1.5 – 1.59 6.8 mL 3 107 mL 162 mL 1.4 – 1.49 6.4 mL 3 107 mL 162 mL 1.30 – 1.39 6 mL 3 108 mL 162 mL 1.20 – 1.29 5.4 mL 2 108 mL 162 mL 1.10 – 1.19 5 mL 2 109 mL 162 mL 1 – 1.09 4.6 mL 2 109 mL 162 mL 0.9 – 0.99 4.2 mL 2 110 mL 162 mL 0.8 – 0.89 3.8 mL 2 110 mL 162 mL 0.7 – 0.79 3.2 mL 2 111 mL 162 mL 0.6 – 0.69 2.8 mL 1 111 mL 162 mL 0.5 – 0.59 2.3 mL 1 111 mL 162 mL 0.4 – 0.49 2 mL 1 112 mL 162 mL 0.35 – 0.39 1.7 mL 1 112 mL 162 mL 0.3 – 0.34 1.4 mL 1 112 mL 162 mL 0.25 – 0.29 1.2 mL 1 113 mL 162 mL 96 hours 15 mcg/m2/day 1.5 – 1.59 8.4 mL 3 132 mL 200 mL 1.4 – 1.49 7.8 mL 3 132 mL 200 mL 1.30 – 1.39 7.4 mL 3 133 mL 200 mL 1.20 – 1.29 6.8 mL 3 133 mL 200 mL 1.10 – 1.19 6.2 mL 3 134 mL 200 mL 1 – 1.09 5.6 mL 2 134 mL 200 mL 0.9 – 0.99 5.2 mL 2 135 mL 200 mL 0.8 – 0.89 4.6 mL 2 135 mL 200 mL 0.7 – 0.79 4 mL 2 136 mL 200 mL 0.6 – 0.69 3.4 mL 2 137 mL 200 mL 0.5 – 0.59 2.8 mL 1 137 mL 200 mL 0.4 – 0.49 2.4 mL 1 138 mL 200 mL 0.35 – 0.39 2.1 mL 1 138 mL 200 mL 0.3 – 0.34 1.8 mL 1 138 mL 200 mL 0.25 – 0.29 1.5 mL 1 139 mL 200 mL 7 days 15 mcg/m2/day 1.5 – 1.59 14 mL 5 3.8 mL 110 mL 1.4 – 1.49 13.1 mL 5 4.7 mL 110 mL 1.30 – 1.39 12.2 mL 5 5.6 mL 110 mL 1.20 – 1.29 11.3 mL 5 6.5 mL 110 mL 1.10 – 1.19 10.4 mL 4 7.4 mL 110 mL 1 – 1.09 9.5 mL 4 8.3 mL 110 mL 0.9 – 0.99 8.6 mL 4 9.2 mL 110 mL 0.8 – 0.89 7.7 mL 3 10.1 mL 110 mL 0.7 – 0.79 6.8 mL 3 11 mL 110 mL 0.6 – 0.69 5.9 mL 3 11.9 mL 110 mL 0.5 – 0.59 5 mL 2 12.8 mL 110 mL 0.4 – 0.49 4.1 mL 2 13.7 mL 110 mL 0.35 – 0.39 3.4 mL 2 14.4 mL 110 mL 0.3 – 0.34 2.8 mL 1 15 mL 110 mL 0.25 – 0.29 2.5 mL 1 15.3 mL 110 mL The administration of BLINCYTO as a 72-hour, 96-hour, and 7-day infusion is not recommended for patients weighing less than 5.4 kg. Administration of BLINCYTO: 72-Hour, 96-Hour, or 7-Day Intravenous Bag - Administer BLINCYTO as a continuous intravenous infusion at a constant flow rate using an infusion pump. The pump should be programmable, lockable, non-elastomeric, and have an alarm.
- The final volume of infusion solution will be more than the volume administered to the patient to account for the priming of the intravenous tubing and to ensure that the patient will receive the full dose of BLINCYTO.
- -
- For 72-hour infusion (162 mL) will be more than the volume administered to the patient (130 mL).
- -
- For 96-hour infusion (200 mL) will be more than the volume administered to the patient (173 mL).
- -
- For 7-day infusion (110 mL) will be more than the volume administered to the patient (100 mL).
- Ensure that the intravenous tubing is primed only with the solution in the bag containing the FINAL prepared BLINCYTO solution for infusion.
- Do not use an in-line filter for 72-hour, 96-hour, or 7-day bags.
- Infuse the FINAL prepared BLINCYTO infusion solution according to the instructions on the pharmacy label on the prepared bag at one of the following constant infusion rates:
- -
- Infusion rate of 1.8 mL/hour for a duration of 72 hours or 96 hours, OR
- -
- Infusion rate of 0.6 mL/hour for a duration of 7 days.
- Important Note: Do not flush the BLINCYTO infusion line, especially when changing infusion bags. Flushing when changing bags or at completion of infusion can result in excess dosage and complications thereof. When administering via a multi-lumen venous catheter, infuse BLINCYTO through a dedicated lumen. Before flushing the catheter system, residual amounts of BLINCYTO must be aspirated from the catheter system to avoid bolus administration.
- At the end of the infusion, any remaining solution in the intravenous bag and intravenous tubing should be discarded in accordance with local requirements.
Manufactured by:
Amgen Inc.
One Amgen Center Drive
Thousand Oaks, California 91320-1799
U.S. License No. 1080BLINCYTO® (blinatumomab)
Patent: http://pat.amgen.com/blincyto/
© 2024 Amgen Inc. All rights reserved.
V2Revised: 12/2024
Close -
PRINCIPAL DISPLAY PANEL - Kit PackageAMGEN® 35 - mcg/vial - 1 BLINCYTO® Single-Dose Vial - 1 IV Solution Stabilizer Vial - NDC 55513-160-01 - BLINCYTO® (blinatumomab) for Injection - 35 mcg/vial - For Intravenous Infusion Only - Store refrigerated ...
AMGEN®
35
mcg/vial1 BLINCYTO® Single-Dose Vial
1 IV Solution Stabilizer VialNDC 55513-160-01
BLINCYTO®
(blinatumomab)
for Injection35 mcg/vial
For Intravenous Infusion Only
Store refrigerated at 2°C to 8°C (36°F to 46°F).
Store in carton to protect from light.
DO NOT SHAKE reconstituted solution.
Dispense the enclosed Medication Guide to each patient.No Preservative
Single-Dose Vial –
Discard unused portion.Rx Only
Close
-
INGREDIENTS AND APPEARANCEProduct Information
BLINCYTO blinatumomab kit Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:55513-160 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55513-160-01 1 in 1 PACKAGE 12/18/2014 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 VIAL 3.088 mL Part 2 1 VIAL 10.6 mL Part 1 of 2 BLINCYTO blinatumomab injection, powder, lyophilized, for solution Product Information Item Code (Source) NDC:55513-150 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BLINATUMOMAB (UNII: 4FR53SIF3A) (BLINATUMOMAB - UNII:4FR53SIF3A) BLINATUMOMAB 12.5 ug in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 1.19 mg in 1 mL LYSINE HYDROCHLORIDE (UNII: JNJ23Q2COM) 8.27 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.23 mg in 1 mL TREHALOSE DIHYDRATE (UNII: 7YIN7J07X4) 34 mg in 1 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55513-150-01 3.088 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125557 12/18/2014 Part 2 of 2 IV STABILIZER iv stabilizer solution Product Information Item Code (Source) NDC:55513-155 Route of Administration INTRAVENOUS Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 5.25 mg in 1 mL LYSINE HYDROCHLORIDE (UNII: JNJ23Q2COM) 228.38 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 1 mg in 1 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55513-155-01 10.6 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125557 12/18/2014 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125557 12/18/2014
CloseLabeler - Amgen Inc (039976196)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
BLINCYTO- blinatumomab kit
Number of versions: 21
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Dec 23, 2024 | 38 (current) | download |
| Jul 23, 2024 | 37 | download |
| Jun 25, 2024 | 36 | download |
| Feb 20, 2024 | 35 | download |
| Jun 30, 2023 | 34 | download |
| Nov 1, 2022 | 32 | download |
| May 25, 2022 | 31 | download |
| Mar 7, 2022 | 29 | download |
| Mar 25, 2021 | 28 | download |
| Apr 6, 2020 | 25 | download |
| Nov 18, 2019 | 23 | download |
| May 3, 2019 | 22 | download |
| May 17, 2018 | 20 | download |
| Apr 10, 2018 | 19 | download |
| Dec 14, 2017 | 18 | download |
| Jul 25, 2017 | 15 | download |
| May 17, 2017 | 14 | download |
| Nov 14, 2016 | 11 | download |
| Sep 14, 2016 | 10 | download |
| Feb 23, 2015 | 7 | download |
| Dec 18, 2014 | 5 | download |
RxNorm
BLINCYTO- blinatumomab kit
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 1597262 | blinatumomab 35 MCG Injection | PSN |
| 2 | 1597262 | blinatumomab 0.035 MG Injection | SCD |
| 3 | 1597262 | blinatumomab 35 MCG Injection | SY |
| 4 | 1597267 | Blincyto 35 MCG Injection | PSN |
| 5 | 1597267 | blinatumomab 0.035 MG Injection [Blincyto] | SBD |
| 6 | 1597267 | Blincyto 0.035 MG Injection | SY |
| 7 | 1597267 | Blincyto 35 MCG Injection | SY |
Get Label RSS Feed for this Drug
BLINCYTO- blinatumomab kit
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=38b482a8-960b-4591-9857-5031ecb830aa
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
BLINCYTO- blinatumomab kit
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 55513-150-01 |
| 2 | 55513-155-01 |
| 3 | 55513-160-01 |