Label: ALLERNAZE- triamcinolone acetonide spray, metered
-
Contains inactivated NDC Code(s)
NDC Code(s): 27437-143-01 - Packager: Lupin Pharmaceuticals
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 23, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONFor Intranasal Use Only
-
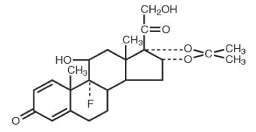
DESCRIPTION:Triamcinolone acetonide, the active ingredient of AllerNaze, is a corticosteroid with the chemical name, 9α-Fluoro-11β,16α, 17, 21-tetrahydroxypregna-1,4-diene-3, 20-dione cyclic 16, 17-acetal ...
-
CLINICAL PHARMACOLOGY:Triamcinolone acetonide is a more potent derivative of triamcinolone. Triamcinolone acetonide is approximately eight times more potent than prednisone in animal models of inflammation. The ...
-
CLINICAL TRIALS:The efficacy of triamcinolone acetonide solution has been evaluated in 746 patients with seasonal or perennial allergic rhinitis who completed 8 controlled clinical trials. In total, 1187 ...
-
INDICATIONS AND USAGE:AllerNaze is indicated for the treatment of the nasal symptoms of seasonal and perennial allergic rhinitis in adults and children 12 years of age or older.
-
CONTRAINDICATIONS:AllerNaze is contraindicated in patients with a hypersensitivity to any of its ingredients.
-
WARNINGS:The replacement of a systemic corticosteroid with a topical corticosteroid can be accompanied by signs of adrenal insufficiency and, in addition, some patients may experience symptoms of ...
-
PRECAUTIONS:General: Intranasal corticosteroids may cause a reduction in growth velocity when administered to pediatric patients (see PRECAUTIONS, Pediatric Use section). In clinical studies with ...
-
ADVERSE REACTIONS:In adequate, well-controlled and uncontrolled studies, 1187 patients have received triamcinolone acetonide solution. The adverse reactions summarized below, are based upon seven placebo controlled ...
-
OVERDOSAGE:Like any other nasally administered corticosteroid, acute overdosing is unlikely in view of the total amount of active ingredient present. In the event that the entire contents of the bottle were ...
-
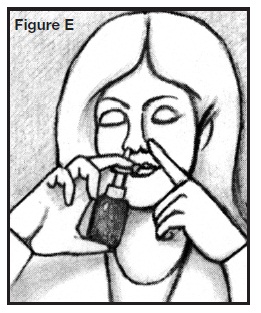
DOSAGE AND ADMINISTRATION:The recommended starting dose of AllerNaze for most patients is 200 mcg per day given as 2 sprays (approximately 50 mcg/spray) in each nostril once a day. The maximum dose should not exceed 400 ...
-
HOW SUPPLIED:Each 15 mL bottle of AllerNaze (NDC 27437-143-01) contains 7.5 mg (0.50 mg/mL) of triamcinolone acetonide, USP and is fitted with a meter pump with white nasal applicator, teal blue dust cover and ...
-
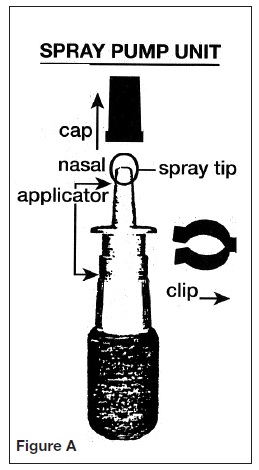
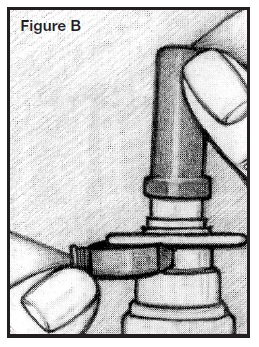
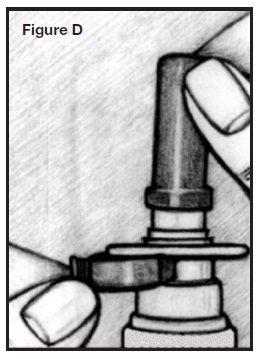
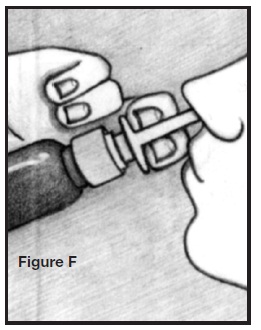
PATIENT’S INSTRUCTIONS FOR USEINFORMATION FOR THE PATIENT - These instructions provide a summary of important information about AllerNaze. Please read it carefully before use. Ask your doctor or pharmacist if you have any ...
-
PACKAGE/LABEL PRINCIPAL DISPLAY PANELNDC 27437-143-01 - AllerNaze™ (triamcinolone acetonide, USP) NASAL SPRAY, 50mcg - Lupin Pharma
-
INGREDIENTS AND APPEARANCEProduct Information