Label: AIRSUPRA- albuterol sulfate and budesonide aerosol, metered
- NDC Code(s): 0310-9080-12, 0310-9080-28
- Packager: AstraZeneca Pharmaceuticals LP
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated March 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use AIRSUPRA® safely and effectively. See full prescribing information for AIRSUPRA. AIRSUPRA (albuterol and budesonide) inhalation ...These highlights do not include all the information needed to use AIRSUPRA® safely and effectively. See full prescribing information for AIRSUPRA.
AIRSUPRA (albuterol and budesonide) inhalation aerosol, for oral inhalation use
Initial U.S. Approval: 2023INDICATIONS AND USAGE
AIRSUPRA is a combination of albuterol, a beta2-adrenergic agonist and budesonide, a corticosteroid, indicated for the as-needed treatment or prevention of bronchoconstriction and to reduce the risk of exacerbations in patients with asthma 18 years of age and older. (1)
DOSAGE AND ADMINISTRATION
- •
- Recommended Dosage: AIRSUPRA 180 mcg/160 mcg (administered as 2 actuations of albuterol/budesonide 90 mcg/80 mcg) by oral inhalation as needed for asthma symptoms. (2.1)
- •
- Do not take more than 6 doses (12 inhalations) in a 24-hour period. (2.1)
- •
- Prime inhaler prior to first use. Re-prime when inhaler has not been used for more than 7 days, is dropped, or after cleaning. (2.2)
- •
- Discard when the dose counter displays 0. (2.3)
DOSAGE FORMS AND STRENGTHS
Inhalation aerosol: Pressurized metered dose inhaler that delivers a combination of albuterol 90 mcg and budesonide 80 mcg per actuation. (3)
CONTRAINDICATIONS
Hypersensitivity to albuterol, budesonide, or to any of the excipients. (4)
WARNINGS AND PRECAUTIONS
- •
- If symptoms continue after using AIRSUPRA, this may be a marker of destabilization of asthma and requires reevaluation of treatment. (5.1)
- •
- If paradoxical bronchospasm occurs, discontinue AIRSUPRA immediately and institute alternative therapy. (5.2)
- •
- Cardiovascular effects may occur. Use with caution in patients sensitive to sympathomimetic drugs and patients with cardiovascular disorders. (5.3)
- •
- Excessive use may be fatal. Do not exceed maximum recommended dosage. (5.4)
- •
- Hypersensitivity reactions may occur. Discontinue AIRSUPRA immediately. (5.5)
- •
- Use with caution in patients with convulsive disorders, hyperthyroidism, diabetes mellitus, and ketoacidosis. (5.6)
- •
- Hypokalemia may occur. (5.7)
- •
- Potential worsening of infections (e.g., existing tuberculosis; fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex). Use with caution in patients with these infections. More serious or even fatal course of chickenpox or measles can occur in susceptible patients. (5.8)
- •
- Oropharyngeal candidiasis may occur. Advise patients to rinse mouth with water, if available, without swallowing after use. Monitor patients periodically. (5.9)
- •
- Hypercorticism and adrenal suppression may occur with very high dosages in susceptible individuals. If such changes occur, consider appropriate therapy. (5.10)
- •
- Monitor patients with major risk factors for decreased bone mineral content. (5.11)
- •
- Glaucoma and cataracts may occur. Consider referral to an ophthalmologist in patients who develop ocular symptoms. (5.12)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 1%) are headache, oral candidiasis, cough, dysphonia (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AstraZeneca at 1-800-236-9933 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- •
- Strong cytochrome P450 3A4 inhibitors (e.g. ritonavir): Use with caution. May cause systemic corticosteroid effects. (7.1)
- •
- Other short-acting bronchodilators: Use judiciously with other short-acting beta agonists. (7.2)
- •
- Beta blockers: May decrease effectiveness of AIRSUPRA and produce severe bronchospasm. When there are no acceptable alternatives to the use of beta-adrenergic-blocking agents, consider cardioselective beta-blockers and use with caution. (7.3)
- •
- Diuretics, or non-potassium-sparing diuretics: May potentiate hypokalemia or ECG changes. Consider monitoring potassium levels with concomitant use. (7.4)
- •
- Digoxin: May decrease serum digoxin levels. Carefully evaluate digoxin levels with concomitant use. (7.5)
- •
- Monoamine oxidase inhibitors (MAOI) and tricyclic antidepressants: Use AIRSUPRA with extreme caution with concomitant use. (7.6)
USE IN SPECIFIC POPULATIONS
Hepatic impairment: Budesonide systemic exposure may increase in patients with severe hepatic impairment. Monitor patients with hepatic disease. (8.6)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2024
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage and Administration
2.2 Priming Before Use
2.3 Dose Counter
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Deterioration of Asthma
5.2 Paradoxical Bronchospasm
5.3 Cardiovascular Effects
5.4 Do Not Exceed Recommended Dosage
5.5 Hypersensitivity Reactions, Including Anaphylaxis
5.6 Risk of Sympathomimetic Amines with Certain Coexisting Conditions
5.7 Hypokalemia
5.8 Immunosuppression and Risk of Infections
5.9 Oropharyngeal Candidiasis
5.10 Hypercorticism and Adrenal Suppression
5.11 Reduction in Bone Mineral Density
5.12 Glaucoma and Cataracts
5.13 Drug Interactions with Strong Cytochrome P450 3A4 Inhibitors
5.14 Effects on Growth in Pediatric Patients
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Inhibitors of Cytochrome P450 3A4
7.2 Use with Other Short-Acting Bronchodilators
7.3 Beta-Blockers
7.4 Diuretics
7.5 Digoxin
7.6 Monoamine Oxidase Inhibitors or Tricyclic Antidepressants
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE AIRSUPRA is indicated for the as-needed treatment or prevention of bronchoconstriction and to reduce the risk of exacerbations in patients with asthma 18 years of age and older.
AIRSUPRA is indicated for the as-needed treatment or prevention of bronchoconstriction and to reduce the risk of exacerbations in patients with asthma 18 years of age and older.
Close -
2 DOSAGE AND ADMINISTRATION 2.1 Recommended Dosage and Administration - The recommended dosage of AIRSUPRA is albuterol 180 mcg and budesonide 160 mcg (administered as 2 actuations of AIRSUPRA [albuterol/budesonide 90 ...
2.1 Recommended Dosage and Administration
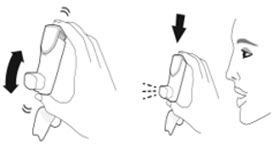
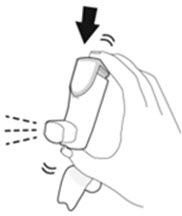
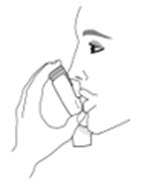
The recommended dosage of AIRSUPRA is albuterol 180 mcg and budesonide 160 mcg (administered as 2 actuations of AIRSUPRA [albuterol/budesonide 90 mcg/80 mcg]) as needed for asthma symptoms by oral inhalation. Do not take more than 6 doses (12 inhalations) in a 24-hour period [see Warnings and Precautions (5.4)].
2.2 Priming Before Use
- •
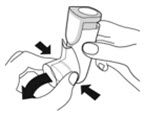
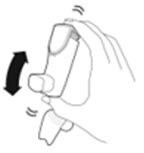
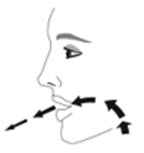
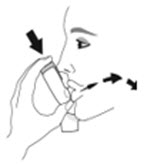
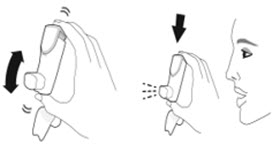
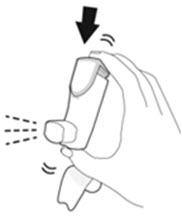
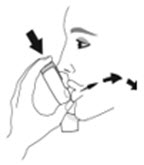
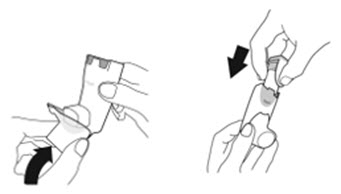
- Priming AIRSUPRA is essential to ensure appropriate drug content in each actuation. Prime AIRSUPRA before using for the first time. To prime AIRSUPRA, release 4 sprays into the air away from the face, shaking well before each spray.
- •
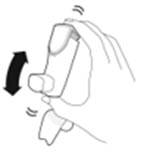
- AIRSUPRA must be re-primed when the inhaler has not been used for more than 7 days, is dropped, or after cleaning. To re-prime AIRSUPRA, release 2 sprays into the air away from the face, shaking well before each spray.
Close2.3 Dose Counter
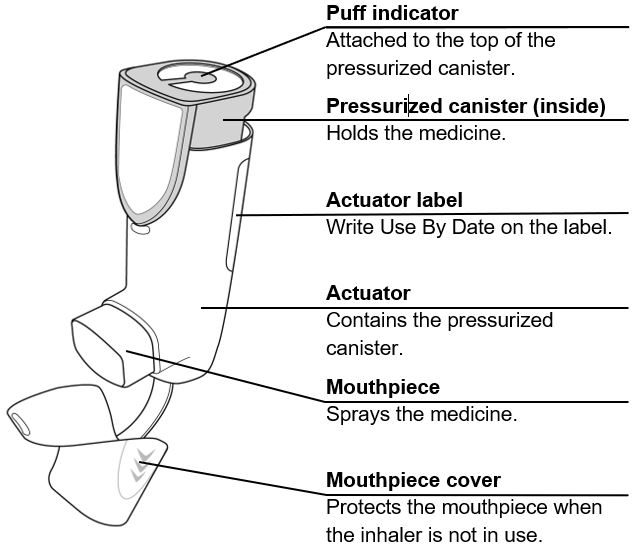
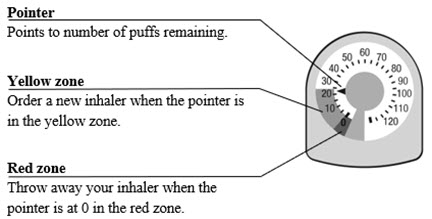
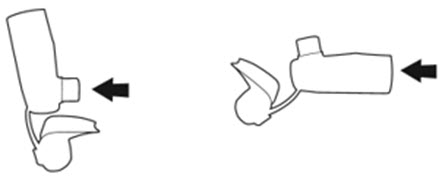
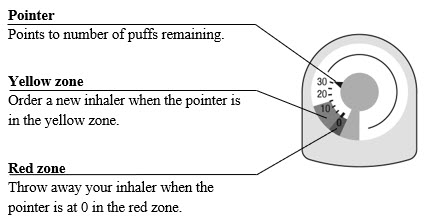
The canister has an attached dose indicator (also known as puff indicator) which indicates how many inhalations (puffs) remain. The dose indicator pointer will move after every actuation. The pointer will show the number of inhalations remaining in the canister. When nearing the end of the usable inhalations, the color behind the number in the dose indicator display window changes to yellow. AIRSUPRA should be discarded when the pointer reaches zero.
-
3 DOSAGE FORMS AND STRENGTHS Inhalation aerosol: a pressurized metered dose inhaler (a pressurized canister with an attached dose indicator, supplied with a white plastic actuator with a frosted clear plastic dust cap) that ...
Inhalation aerosol: a pressurized metered dose inhaler (a pressurized canister with an attached dose indicator, supplied with a white plastic actuator with a frosted clear plastic dust cap) that delivers a combination of albuterol 90 mcg and budesonide 80 mcg per actuation.
Close -
4 CONTRAINDICATIONS AIRSUPRA is contraindicated in patients with a history of hypersensitivity to albuterol, budesonide, or any of the excipients [see Warnings and Precautions (5.5), Description (11)].
AIRSUPRA is contraindicated in patients with a history of hypersensitivity to albuterol, budesonide, or any of the excipients [see Warnings and Precautions (5.5), Description (11)].
Close -
5 WARNINGS AND PRECAUTIONS 5.1 Deterioration of Asthma - Asthma may deteriorate acutely over a period of hours or chronically over several days or longer. If the patient continues to experience symptoms after using ...
5.1 Deterioration of Asthma
Asthma may deteriorate acutely over a period of hours or chronically over several days or longer. If the patient continues to experience symptoms after using AIRSUPRA or requires more doses of AIRSUPRA than usual, this may be a marker of destabilization of asthma and requires evaluation of the patient and their treatment regimen.
5.2 Paradoxical Bronchospasm
AIRSUPRA can produce paradoxical bronchospasm, which may be life threatening. If paradoxical bronchospasm occurs following dosing with AIRSUPRA, it should be discontinued immediately, and alternative therapy should be instituted. It should be recognized that paradoxical bronchospasm, when associated with inhaled formulations, frequently occurs with the first use of a new canister.
5.3 Cardiovascular Effects
AIRSUPRA, like other drugs containing beta2-adrenergic agonists, can produce clinically significant cardiovascular effects in some patients as measured by increases in pulse rate, blood pressure, and/or other symptoms. If such effects occur, AIRSUPRA may need to be discontinued. In addition, beta-agonists have been reported to produce electrocardiogram (ECG) changes, such as flattening of the T wave, prolongation of the QTc interval, and ST-segment depression. The clinical significance of these findings is unknown. Therefore, AIRSUPRA, like all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
5.4 Do Not Exceed Recommended Dosage
As with other inhaled drugs containing beta-adrenergic agents, AIRSUPRA should not be used more than the maximum daily dose [see Dosage and Administration (2.1)], as an overdose may result. Clinically significant cardiovascular effects and fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs.
5.5 Hypersensitivity Reactions, Including Anaphylaxis
Hypersensitivity reactions can occur after administration of albuterol sulfate and budesonide, components of AIRSUPRA, as demonstrated by cases of anaphylaxis, angioedema, bronchospasm, oropharyngeal edema, rash, and urticaria. Discontinue AIRSUPRA if such reactions occur [see Contraindications (4)].
5.6 Risk of Sympathomimetic Amines with Certain Coexisting Conditions
AIRSUPRA, like all therapies containing sympathomimetic amines, should be used with caution in patients with convulsive disorders, hyperthyroidism, or diabetes mellitus and in patients who are unusually responsive to sympathomimetic amines. Large doses of intravenous albuterol have been reported to aggravate preexisting diabetes mellitus and ketoacidosis.
5.7 Hypokalemia
Beta-adrenergic agonist medicines may produce significant hypokalemia in some patients, possibly through intracellular shunting, which has the potential to produce adverse cardiovascular effects [see Warnings and Precautions (5.3), Clinical Pharmacology (12.1)]. The decrease in serum potassium is usually transient, not requiring supplementation.
5.8 Immunosuppression and Risk of Infections
Patients who are using drugs that suppress the immune system are more susceptible to infection. Chicken pox and measles, for example, can have a more serious or even fatal course in susceptible patients using corticosteroids. In patients who have not had these diseases or been properly immunized, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affects the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If a patient is exposed to chicken pox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated (see the respective Prescribing Information for VZIG and IG). If chicken pox develops, treatment with antiviral agents may be considered.
Inhaled corticosteroids should be used with caution, if at all, in patients with active or quiescent tuberculosis infection of the respiratory tract; untreated systemic fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex.
5.9 Oropharyngeal Candidiasis
AIRSUPRA contains budesonide, an inhaled corticosteroid (ICS). Localized infections of the mouth and pharynx with Candida albicans have occurred in patients treated with ICS agents. Monitor patients periodically. When such an infection develops, it should be treated with appropriate local or systemic (i.e., oral) antifungal therapy while treatment with AIRSUPRA continues. In some cases, therapy with AIRSUPRA may need to be interrupted. Advise the patient to rinse his/her mouth with water, if available, without swallowing following administration of AIRSUPRA to help reduce the risk of oropharyngeal candidiasis.
5.10 Hypercorticism and Adrenal Suppression
Budesonide, a component of AIRSUPRA, will often help control asthma symptoms with less suppression of hypothalamic-pituitary-adrenal (HPA) function than therapeutically equivalent oral doses of prednisone. Since budesonide is absorbed into the circulation and can be systemically active at higher doses, the beneficial effects of AIRSUPRA in minimizing HPA dysfunction may be expected only when recommended dosages are not exceeded. Since individual sensitivity to effects on cortisol production exists, physicians should consider this information when prescribing AIRSUPRA.
Because of the possibility of systemic absorption of ICS, patients treated with AIRSUPRA should be observed carefully for any evidence of systemic corticosteroid effects. Particular care should be taken in observing patients postoperatively or during periods of stress for evidence of inadequate adrenal response.
It is possible that systemic corticosteroid effects such as hypercorticism and adrenal suppression (including adrenal crisis) may appear in a small number of patients who are sensitive to these effects. If such effects occur, appropriate therapy should be initiated as needed.
5.11 Reduction in Bone Mineral Density
Decreases in bone mineral density (BMD) have been observed with long-term administration of products containing ICS. The clinical significance of small changes in BMD with regard to long-term consequences such as fracture is unknown. Patients with major risk factors for decreased bone mineral content, such as prolonged immobilization, family history of osteoporosis, post-menopausal status, tobacco use, advanced age, poor nutrition, or chronic use of drugs that can reduce bone mass (e.g., anticonvulsants, oral corticosteroids) should be monitored and treated with established standards of care.
5.12 Glaucoma and Cataracts
Glaucoma, increased intraocular pressure, and cataracts have been reported following the long-term administration of ICS, including budesonide, a component of AIRSUPRA. Consider referral to an ophthalmologist in patients who develop ocular symptoms.
5.13 Drug Interactions with Strong Cytochrome P450 3A4 Inhibitors
Caution should be exercised when considering the co-administration of AIRSUPRA with long-term ketoconazole and other known strong CYP3A4 inhibitors (e.g., ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, telithromycin) because adverse effects related to increased systemic exposure to budesonide may occur [see Drug Interactions (7.1), Clinical Pharmacology (12.3)].
Close5.14 Effects on Growth in Pediatric Patients
Orally inhaled corticosteroids, including budesonide, may cause a reduction in growth velocity when administered to pediatric patients. The safety and effectiveness of AIRSUPRA have not been established in pediatric patients, and AIRSUPRA is not indicated for use in this population [see Use in Specific Populations (8.4)].
-
6 ADVERSE REACTIONS The following clinically significant adverse reactions are described elsewhere in labeling: • Deterioration of Asthma [see Warnings and Precautions (5.1)] • Paradoxical Bronchospasm [see ...
The following clinically significant adverse reactions are described elsewhere in labeling:
- •
- Deterioration of Asthma [see Warnings and Precautions (5.1)]
- •
- Paradoxical Bronchospasm [see Warnings and Precautions (5.2)]
- •
- Cardiovascular Effects [see Warnings and Precautions (5.3)]
- •
- Hypersensitivity Reactions, including Anaphylaxis [see Warnings and Precautions (5.5)]
- •
- Hypokalemia [see Warnings and Precautions (5.7)]
- •
- Immunosuppression and Risk of Infections [see Warnings and Precautions (5.8)]
- •
- Oropharyngeal Candidiasis [see Warnings and Precautions (5.9)]
- •
- Hypercorticism and Adrenal Suppression [see Warnings and Precautions (5.10)]
- •
- Reduction in Bone Mineral Density [see Warnings and Precautions (5.11)]
- •
- Glaucoma and Cataracts [see Warnings and Precautions (5.12)]
- •
- Effects on Growth in Pediatric Patients [see Warnings and Precautions (5.14)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of AIRSUPRA is based on data from 2 trials: MANDALA and DENALI [see Clinical Studies (14)]. All reported safety data is based on patients who received AIRSUPRA 180 mcg/160 mcg.
In MANDALA, in which patients with moderate to severe asthma were administered AIRSUPRA as needed, a total of 1015 patients 12 to 84 years of age (mean 51 years) received at least one dose of AIRSUPRA and participated in the study for a mean duration of 310 days. Of these, 905 patients were exposed for at least 24 weeks and 323 patients had exposure for at least 1 year. The mean daily use was 2.6 actuations. On the majority of study days, patients used 2 or less actuations; more than 8 actuations were used on less than 2% of study days. AIRSUPRA is not approved for patients 12 to 17 years of age.
The incidence of common adverse reactions in MANDALA is described in Table 1.
Table 1 Summary of Adverse Reactions with AIRSUPRA Reported in ≥ 1% of Patients (MANDALA) Adverse Reaction
AIRSUPRA
180 mcg/160 mcg
N = 1015 (%)AS MDI1
180 mcg
N = 1015 (%)Headache
44 (4.3)
50 (4.9)
Oral candidiasis2
13 (1.3)
5 (0.5)
Cough
10 (1.0)
11 (1.1)
1 Albuterol Metered Dose Inhaler = AS MDI
2 Oral candidiasis also includes those reactions reported under the preferred term oropharyngeal candidiasis.The safety profile of AIRSUPRA in MANDALA was similar across the 2 treatment groups irrespective of background ICS dose.
In DENALI, AIRSUPRA 180 mcg/160 mcg was administered 4 times a day for 12 weeks. A total of 197 patients 13 to 81 years of age (mean 50 years) with mild to moderate asthma received at least 1 dose of AIRSUPRA. The adverse reactions profile was similar to MANDALA except for the following adverse reactions for AIRSUPRA with an incidence ≥ 1.0% that exceeded the incidence in MANDALA: headache (5.1%), dysphonia (2.0%), and oral/oropharyngeal candidiasis (1.5%), compared to headache (7.1%), dysphonia (0%), and oral/oropharyngeal candidiasis (0%) in the placebo arm.
Close6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of budesonide or albuterol. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular disorders: myocardial ischemia, tremor, tachycardia, palpitations, extrasystoles, arrhythmias (including atrial fibrillation, supraventricular tachycardia), syncope, hypertension, peripheral vasodilatation
Endocrine disorders: signs or symptoms of systemic glucocorticosteroid effect (including hypofunction of the adrenal gland and reduction of growth rate)
Eye disorders: cataracts, glaucoma, increased intraocular pressure
Immune system disorders: immediate and delayed hypersensitivity reactions (including anaphylactic reaction, angioedema, bronchospasm, rash, contact dermatitis and urticaria)
General disorders: fever, weight gain, taste perversion, flu syndrome
Gastrointestinal disorders: nausea, vomiting, dyspepsia, diarrhea
Infections: sinusitis, pharyngitis, respiratory tract infection, nasopharyngitis, gastroenteritis, otitis media, laryngitis
Metabolic disorders: hypokalemia, metabolic acidosis
Musculoskeletal disorders: hypertonia, musculoskeletal pain, myalgia, asthenia, arthralgia, muscle cramps, fracture
Neurological or psychiatric system disorders: migraine, dizziness, central nervous system stimulation, insomnia, hyperactivity, psychiatric symptoms (including psychosis, depression, aggressive reactions, irritability, nervousness, restlessness, behavioral disturbances and anxiety)
Respiratory, thoracic, and mediastinal disorders: rhinitis, nasal congestion, throat irritation, oropharyngeal edema, upper respiratory inflammation, drying or irritation of the oropharynx
Skin and subcutaneous tissue disorders: skin bruising, ecchymosis
-
7 DRUG INTERACTIONS No formal drug interaction studies have been performed with AIRSUPRA. 7.1 Inhibitors of Cytochrome P450 3A4 - The main route of metabolism of corticosteroids, including budesonide, a component ...
No formal drug interaction studies have been performed with AIRSUPRA.
7.1 Inhibitors of Cytochrome P450 3A4
The main route of metabolism of corticosteroids, including budesonide, a component of AIRSUPRA, is via cytochrome P450 (CYP) isoenzyme 3A4 (CYP3A4). After oral administration of ketoconazole, a strong inhibitor of CYP3A4, the mean plasma concentration of orally administered budesonide increased. Concomitant administration of a CYP3A4 inhibitor may inhibit the metabolism of, and increase the systemic exposure to, budesonide. Caution should be exercised when considering the co-administration of AIRSUPRA with long-term ketoconazole and other known strong CYP3A4 inhibitors (e.g., ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, telithromycin) [see Warnings and Precautions (5.11)].
7.2 Use with Other Short-Acting Bronchodilators
AIRSUPRA contains the short-acting beta-agonist, albuterol. Therefore, concomitant use of additional beta-agonists with AIRSUPRA should be used judiciously to prevent beta-agonist overdose.
7.3 Beta-Blockers
Beta-adrenergic receptor blocking agents not only block the pulmonary effect of beta-agonists, such as albuterol, a component of AIRSUPRA, but may also produce severe bronchospasm in patients with asthma. Therefore, patients with asthma should not normally be treated with beta-blockers. However, under certain circumstances, e.g., as prophylaxis after myocardial infarction, there may be no acceptable alternatives to the use of beta-adrenergic-blocking agents in these patients. In this setting, consider cardioselective beta-blockers, although they should be administered with caution.
7.4 Diuretics
The ECG changes and/or hypokalemia which may result from the administration of non-potassium-sparing diuretics (such as loop or thiazide diuretics) can be acutely worsened by beta-agonists, especially when the recommended dose of the beta-agonist is exceeded. Although the clinical significance of these effects is not known, caution is advised in the coadministration of AIRSUPRA with non-potassium-sparing diuretics. Consider monitoring potassium levels.
7.5 Digoxin
Mean decreases of 16% and 22% in serum digoxin levels were demonstrated after single-dose intravenous and oral administration of albuterol, respectively, to normal volunteers who had received digoxin for 10 days. The clinical significance of these findings for patients with obstructive airway disease who are receiving albuterol and digoxin on a chronic basis is unclear. Nevertheless, it would be prudent to carefully evaluate the serum digoxin levels in patients who are currently receiving digoxin and AIRSUPRA.
Close7.6 Monoamine Oxidase Inhibitors or Tricyclic Antidepressants
AIRSUPRA should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors or tricyclic antidepressants, or within 2 weeks of discontinuation of such agents, because the action of albuterol on the cardiovascular system may be potentiated.
-
8 USE IN SPECIFIC POPULATIONS 8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to asthma medications during pregnancy. For more ...
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to asthma medications during pregnancy. For more information, contact the MotherToBaby Pregnancy Studies conducted by the Organization of Teratology Information Specialists at 1-877-311-8972 or visit https://mothertobaby.org/ongoing-study/asthma/.
Risk Summary
Available data from published case series, epidemiological studies and reviews with budesonide use in pregnant women have not identified a drug-related risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. Available data from epidemiological studies and postmarketing case reports of pregnancy outcomes following inhaled albuterol use do not consistently demonstrate a risk of major birth defects or miscarriage. The available epidemiological studies have methodologic limitations, including inconsistent comparator groups, definitions of outcomes, and assessment of disease impact. There are risks to the mother and fetus associated with asthma in pregnancy (see Clinical Considerations). Animal reproduction studies have not been conducted with AIRSUPRA, however, animal studies are available with its individual components, albuterol and budesonide.
Administration of albuterol to mice and rabbits during the period of organogenesis revealed evidence of adverse developmental outcomes (cleft palate in mice, delayed ossification in rabbits) at less than maximum recommended human daily inhalation dose (MRHDID) (see Data).
In animal reproduction studies, budesonide, administered by the subcutaneous route, caused structural abnormalities, was embryocidal, and reduced fetal weights in rats and rabbits at less than the MRHDID in adults, but these effects were not seen in rats that received inhaled doses approximately 2.5 times the MRHDID in adults (see Data). Experience with oral corticosteroids suggests that rodents are more prone to structural abnormalities from corticosteroid exposure than humans.
The background risk of major birth defects and miscarriage of the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal risk
In women with poorly or moderately controlled asthma, there is an increased risk of several perinatal adverse outcomes such as preeclampsia in the mother and prematurity, low birth weight, and small for gestational age in the neonate. Pregnant women with asthma should be closely monitored and medication adjusted as necessary to maintain optimal asthma control.
Labor or Delivery
Because of the potential for beta-agonist interference with uterine contractility, use of AIRSUPRA during labor should be restricted to those patients in whom the benefits clearly outweigh the risk. AIRSUPRA has not been approved for the management of pre-term labor. Serious adverse reactions, including pulmonary edema, have been reported during or following treatment of premature labor with beta2-agonists, including albuterol.
Data
Animal Data
Albuterol
In a study in pregnant mice, subcutaneously administered albuterol sulfate produced cleft palate formation in 5 of 111 (4.5%) fetuses at an exposure less than the MRHDID in adults (on a mg/m2 basis at a maternal dose of 0.25 mg/kg) and in 10 of 108 (9.3%) fetuses at approximately 9 times the MRHDID in adults (on a mg/m2 basis at a maternal dose of 2.5 mg/kg). Cleft palate also occurred in 22 of 72 (30.5%) fetuses from females treated subcutaneously with isoproterenol, another beta2-agonist.In a study in pregnant rabbits, orally administered albuterol sulfate produced cranioschisis in 7 of 19 fetuses (37%) at approximately 750 times the MRHDID in adults (on a mg/m2 basis at a maternal dose of 50 mg/kg).
In a study in pregnant rabbits, an albuterol/HFA-134a formulation administered by inhalation produced enlargement of the frontal portion of the fetal fontanelles at approximately one third of the MRHDID on a mg/m2 basis.
A study in which pregnant rats were dosed with radiolabeled albuterol sulfate demonstrated that drug-related material is transferred from the maternal circulation to the fetus.
Budesonide
In a fertility and reproduction study, male rats were subcutaneously dosed with budesonide for 9 weeks and females for 2 weeks prior to pairing and throughout the mating period. Females were dosed up until weaning of their offspring. Budesonide caused a decrease in prenatal viability and viability in the pups at birth and during lactation, along with a decrease in maternal body-weight gain, at doses 0.2 times the MRHDID in adults (on a mcg/m2 basis at maternal subcutaneous doses of 20 mcg/kg/day and above). No such effects were noted at a dose 0.05 times the MRHDID in adults (on a mcg/m2 basis at a maternal subcutaneous dose of 5 mcg/kg/day).In an embryo-fetal development study in pregnant rabbits dosed during the period of organogenesis from gestation days 6-18, budesonide produced fetal loss, decreased fetal weight, and skeletal abnormalities at doses 0.5 times the MRHDID in adults (on a mcg/m2 basis at a maternal subcutaneous dose of 25 mcg/kg/day). In an embryo-fetal development study in pregnant rats dosed during the period of organogenesis from gestation days 6-15, budesonide produced similar adverse fetal effects at doses approximately 5 times the MRHDID in adults (on a mcg/m2 basis at a maternal subcutaneous dose of 500 mcg/kg/day). In another embryo-fetal development study in pregnant rats, no structural abnormalities or embryocidal effects were seen at doses approximately 2.5 times the MRHDID in adults (on a mcg/m2 basis at maternal inhalation doses up to 250 mcg/kg/day).
In a peri- and post-natal development study, rats were dosed from gestation day 15 to postpartum day 21. Budesonide had no effects on delivery but did have an effect on growth and development of offspring. Offspring survival was reduced and surviving offspring had decreased mean body weights at birth and during lactation at doses 0.2 times the MRHDID in adults and higher (on a mcg/m2 basis at maternal subcutaneous doses of 20 mcg/kg/day and higher). These findings occurred in the presence of maternal toxicity.
8.2 Lactation
Risk Summary
There are no available data on the effects of AIRSUPRA on the breastfed child or on milk production.
There are no available data on the presence of albuterol in human milk, the effects on the breastfed child, or the effects on milk production. However, plasma levels of albuterol after inhaled therapeutic doses are low in humans, and if present in breast milk, are likely to be correspondingly low.
Budesonide, like other inhaled corticosteroids, is present in human milk (see Data).
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for AIRSUPRA and any potential adverse effects on the breastfed child from AIRSUPRA or from the underlying maternal condition.
Data
One published study reports that budesonide is present in human milk following maternal inhalation of budesonide, which resulted in infant doses approximately 0.3% to 1% of the maternal weight-adjusted dosage and a milk to plasma ratio was approximately 0.5. Budesonide was not detected in plasma, and no adverse events were noted in the breastfed infants following maternal use of inhaled budesonide.
For AIRSUPRA, the dose of budesonide available to the infant in breast milk, as a percentage of the maternal dose, would be expected to be similar.
Budesonide levels in plasma samples obtained from five infants at about 90 minutes after breastfeeding (and about 140 minutes after drug administration to the mother) were below quantifiable levels (< 0.02 nmol/L in four infants and < 0.04 nmol/L in one infant).
8.4 Pediatric Use
The safety and effectiveness of AIRSUPRA have not been established in pediatric patients. A limited number of pediatric patients (4 to 17 years of age) were enrolled in the efficacy trial (MANDALA) to evaluate AIRSUPRA to reduce the risk of severe asthma exacerbations. The primary efficacy endpoint was time to first severe asthma exacerbation. Results showed there were 9 patients with severe exacerbation events in 34 patients 12 to 17 years of age treated with AIRSUPRA 180 mcg/160 mcg and 7 patients with severe exacerbation events in 34 patients treated with albuterol (AS MDI) [HR 1.44 (0.54, 3.87)]. There were 11 patients with severe exacerbation events in 41 patients 4 to 11 years of age treated with albuterol/budesonide (AS-BD MDI) 180 mcg/80 mcg and 10 in the 42 patients 4 to 11 years of age treated with AS MDI [HR: 1.09 (0.46, 2.56)]. These data are inadequate to make a determination regarding the safety or effectiveness of AIRSUPRA or AS-BD 180 mcg/80 mcg in pediatric patients 4 to 17 years of age [see Clinical Studies (14)].
Controlled clinical studies have shown that ICS agents, including budesonide, one of the components of AIRSUPRA, may cause a reduction in growth velocity in pediatric patients. The effects of long-term treatment of pediatric patients with ICS on final adult height are not known [see Warnings and Precautions (5.14)].
8.5 Geriatric Use
There were 741 patients 65 years of age and older in the clinical studies for asthma [see Clinical Studies (14)]. Of the total number of AIRSUPRA-treated patients in these studies, 231 (19%) were 65 years of age and older, while 41 (3%) were 75 years of age and older. In general, no differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
As with other products containing beta2-agonists, special caution should be observed when using AIRSUPRA in geriatric patients who have concomitant cardiovascular disease that could be adversely affected by this class of drug.
All beta2-adrenergic agonists, including albuterol, are known to be substantially excreted by the kidney, and the risk of toxic reactions may be greater in patients with impaired renal function. Because geriatric patients are more likely to have decreased renal function, care should be taken when dosing, and it may be useful to monitor renal function.
8.6 Hepatic Impairment
Formal pharmacokinetic studies using AIRSUPRA have not been conducted in patients with hepatic impairment. However, since budesonide is predominantly cleared by hepatic metabolism, impairment of liver function may lead to accumulation of budesonide in the plasma. Therefore, patients with hepatic disease should be closely monitored.
Close8.7 Renal Impairment
Formal pharmacokinetic studies using AIRSUPRA have not been conducted in patients with renal impairment.
-
10 OVERDOSAGE AIRSUPRA contains both albuterol and budesonide; therefore, the risks associated with overdosage for the individual components described below apply to AIRSUPRA. Albuterol - The expected symptoms ...
AIRSUPRA contains both albuterol and budesonide; therefore, the risks associated with overdosage for the individual components described below apply to AIRSUPRA.
Albuterol
The expected symptoms with overdosage are those of excessive beta-adrenergic stimulation and/or occurrence or exaggeration of any of the symptoms of beta-adrenergic stimulation (e.g., seizures, angina, hypertension or hypotension, tachycardia with rates up to 200 beats/minute, arrhythmias, nervousness, headache, tremor, muscle cramps, dry mouth, palpitation, nausea, dizziness, fatigue, malaise, insomnia, hyperglycemia, and metabolic acidosis).
Hypokalemia may also occur. As with all sympathomimetic medications, cardiac arrest and even death may be associated with abuse of AIRSUPRA.
Treatment consists of discontinuation of AIRSUPRA together with appropriate symptomatic therapy. The judicious use of a cardioselective beta-receptor blocker may be considered, bearing in mind that such medication can produce bronchospasm. There is insufficient evidence to determine if dialysis is beneficial for overdosage of AIRSUPRA.
Budesonide
If used at excessive doses for prolonged periods, systemic corticosteroid effects such as hypercorticism may occur [see Warnings and Precautions (5.10)].
Close -
11 DESCRIPTION AIRSUPRA (albuterol and budesonide) Inhalation Aerosol is a pressurized metered dose inhaler containing a combination of micronized albuterol sulfate, a relatively selective, short-acting ...
AIRSUPRA (albuterol and budesonide) Inhalation Aerosol is a pressurized metered dose inhaler containing a combination of micronized albuterol sulfate, a relatively selective, short-acting beta2-adrenergic agonist and micronized budesonide, a corticosteroid, for oral inhalation.
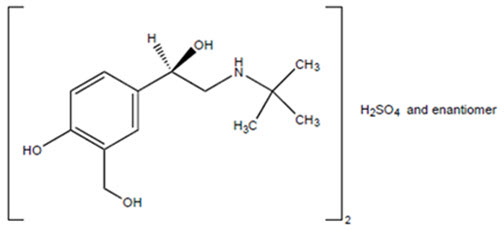
Albuterol sulfate is the official generic name in the United States. The World Health Organization recommended name for the drug is salbutamol sulfate. Albuterol sulfate has the following chemical name: USP racemic α1 [(tert-Butylamino)methyl]-4-hydroxy-m-xylene-α,α'-diol sulfate (2:1) (salt). The empirical formula is (C13H21NO3)2•H2SO4, and the molecular weight is 576.7. Its structural formula is:
Albuterol sulfate is a white to off-white powder that is freely soluble in water, slightly soluble in alcohol and in ether, and practically insoluble in chloroform. It has a LogP of 0.44 at physiological pH. Albuterol has one chiral center and exists as a 1:1 racemic mixture.
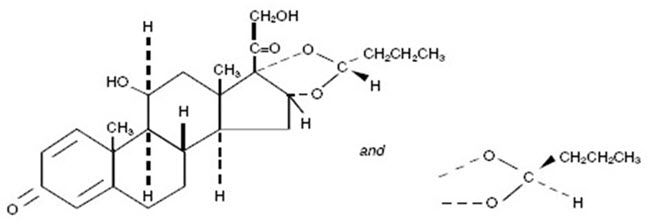
Budesonide is a corticosteroid designated chemically as (RS)-11β, 16α, 17,21-Tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with butyraldehyde. Budesonide is provided as a mixture of two epimers (22R and 22S). The empirical formula of budesonide is C25H34O6 and its molecular weight is 430.5. Its structural formula is:
Budesonide is a white to off-white powder that is practically insoluble in water and in heptane, sparingly soluble in ethanol, and freely soluble in chloroform. Its partition coefficient between octanol and water at pH 7.4 is 1.6 x 103.
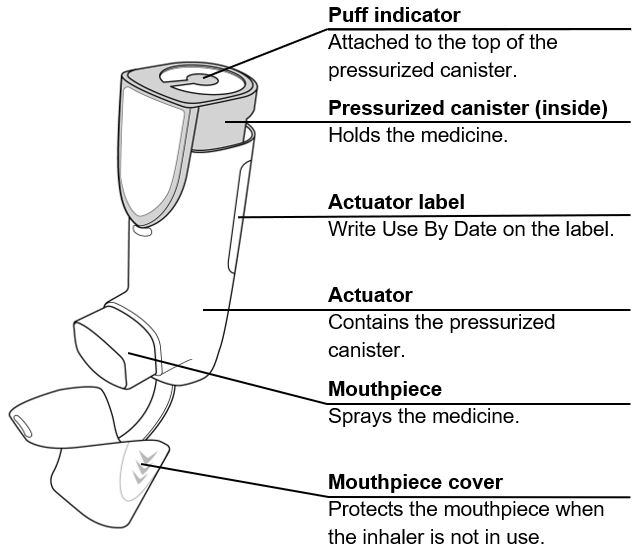
AIRSUPRA is formulated as a hydrofluoroalkane (HFA-134a) propelled pressurized metered dose inhaler containing 120 inhalations. The canister has an attached dose indicator and is supplied with a white plastic actuator with a frosted clear plastic dust cap tethered to it.
After priming, each actuation of AIRSUPRA 90 mcg/80 mcg meters 99 mcg of albuterol and 88 mcg of budesonide from the valve which delivers 90 mcg of albuterol (equivalent to 108 mcg of albuterol sulfate) and 80 mcg of budesonide from the actuator. The actual amount of drug delivered to the lung may depend on patient factors, such as the coordination between actuation of the device and inhalation through the delivery system.
AIRSUPRA also contains porous particles that form a co-suspension with the drug crystals. The porous particles are comprised of the phospholipid 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) and calcium chloride. Porous particles and HFA-134a are excipients in the formulation.
Close -
12 CLINICAL PHARMACOLOGY 12.1 Mechanism of Action - AIRSUPRA contains both albuterol and budesonide; therefore, the mechanisms of action described below for the individual components apply to AIRSUPRA. These drugs ...
12.1 Mechanism of Action
AIRSUPRA contains both albuterol and budesonide; therefore, the mechanisms of action described below for the individual components apply to AIRSUPRA. These drugs represent two classes of medications (a short-acting selective beta2-adrenergic agonist and a synthetic corticosteroid) that have different effects on clinical, physiological, and inflammatory indices of asthma.
Albuterol
In vitro studies and in vivo pharmacologic studies have demonstrated that albuterol has a preferential effect on beta2-adrenergic receptors compared with isoproterenol. Although beta2-adrenoceptors are the predominant adrenergic receptors in bronchial smooth muscle and beta1-adrenoceptors are the predominant receptors in the heart, there are also beta2-adrenoceptors in the human heart comprising 10% to 50% of the total beta-adrenoceptors. The precise function of these receptors has not been established, but their presence raises the possibility that even selective beta2-agonists may have cardiac effects.
Activation of beta2-adrenergic receptors on airway smooth muscle leads to the activation of adenyl cyclase and to an increase in the intracellular concentration of cyclic-3′,5′-adenosine monophosphate (cyclic AMP). This increase of cyclic AMP leads to the activation of protein kinase A, which inhibits the phosphorylation of myosin and lowers intracellular ionic calcium concentrations, resulting in relaxation. Albuterol relaxes the smooth muscles of all airways, from the trachea to the terminal bronchioles. Albuterol acts as a functional antagonist to relax the airway irrespective of the spasmogen involved, thus protecting against all bronchoconstrictor challenges. Increased cyclic AMP concentrations are also associated with the inhibition of release of mediators from mast cells in the airway.
Albuterol has been shown in most controlled clinical trials to have more effect on the respiratory tract, in the form of bronchial smooth muscle relaxation, than isoproterenol at comparable doses while producing fewer cardiovascular effects. Controlled clinical studies and other clinical experience have shown that inhaled albuterol, like other beta-adrenergic agonist drugs, can produce a significant cardiovascular effect in some patients, as measured by pulse rate, blood pressure, symptoms, and/or electrocardiographic changes [see Warnings and Precautions (5.3)].
Budesonide
Budesonide is an anti-inflammatory corticosteroid that exhibits potent glucocorticoid activity and weak mineralocorticoid activity. In standard in vitro and animal models, budesonide has approximately a 200-fold higher affinity for the glucocorticoid receptor and a 1000-fold higher topical anti-inflammatory potency than cortisol (rat croton oil ear edema assay). As a measure of systemic activity, budesonide is 40 times more potent than cortisol when administered subcutaneously and 25 times more potent when administered orally in the rat thymus involution assay. The clinical significance of this is unknown.
In glucocorticoid receptor affinity studies, the 22R form was two times as active as the 22S epimer. In vitro studies indicated that the two forms of budesonide do not interconvert.
The precise mechanism of corticosteroid actions on inflammation in asthma is not known. Inflammation is an important component in the pathogenesis of asthma. Corticosteroids have a wide range of inhibitory activities against multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines) involved in allergic and non-allergic-mediated inflammation. These anti-inflammatory actions of corticosteroids may contribute to their efficacy in asthma.
Studies in asthmatic patients have shown a favorable ratio between topical anti-inflammatory activity and both genomic and non-genomic systemic corticosteroid effects over a wide range of doses of inhaled budesonide. This is explained by a combination of a relatively high local anti-inflammatory effect, extensive first pass hepatic degradation of orally absorbed drug (85-95%), and the low potency of formed metabolites (see below).
12.2 Pharmacodynamics
Albuterol
Albuterol has been shown in most controlled clinical trials to have more effect on the respiratory tract in the form of bronchial smooth muscle relaxation than isoproterenol at comparable doses while producing fewer cardiovascular effects. Controlled clinical studies and other clinical experience have shown that inhaled albuterol, like other beta-adrenergic agonist drugs, can produce a significant cardiovascular effect in some patients, as measured by pulse rate, blood pressure, symptoms, and/or electrocardiographic changes [see Warnings and Precautions (5.3)].
Budesonide
The therapeutic effects of conventional doses of orally inhaled budesonide are largely explained by its direct local action on the respiratory tract.
Inhaled budesonide has been shown to decrease airway reactivity in various challenge models, including histamine, methacholine, sodium metabisulfite, and adenosine monophosphate in patients with hyperreactive airways. The clinical relevance of these models is not certain.
HPA Axis Effects
AIRSUPRA, like other inhaled corticosteroid products, may impact the HPA axis, especially in susceptible individuals, in younger children, and in patients given high doses for prolonged periods [see Warnings and Precautions (5.10)].
The effects of AIRSUPRA on hypothalamic-pituitary-adrenal (HPA) axis were not studied in dedicated clinical trials.
The effects of inhaled AIRSUPRA 180 mcg/160 mcg and AS-BD MDI 180 mcg/80 mcg administered as 2 inhalations of 90 mcg/80 mcg or 90 mcg/40 mcg as needed in response to symptoms were studied on morning serum cortisol in 1254 patients in MANDALA with moderate to severe asthma between the ages of 4 and 84 years. Morning serum cortisol was collected at baseline, Week 24, end of study, or time of discontinuation. Morning cortisol concentration changes over time varied across treatment groups with mean changes from baseline to the Week 24 visit of ‑1.52 nmol/L, +5.43 nmol/L, and +15.42 nmol/L in the AIRSUPRA 180 mcg/160 mcg, AS-BD MDI 180 mcg/80 mcg, and AS MDI 180 mcg treatment groups, respectively.
Close12.3 Pharmacokinetics
The pharmacokinetics of AIRSUPRA and its individual components were evaluated in 3 studies in subjects 18 years of age and older (2 studies in healthy subjects and 1 study in patients with asthma). The pharmacokinetics of albuterol and budesonide from AIRSUPRA are comparable to the pharmacokinetics of albuterol and budesonide when administered as single dose of the individual components in studies of healthy subjects.
The pharmacokinetics presented below are primarily from studies conducted on AIRSUPRA and on its individual components, albuterol (AS MDI) and budesonide (BD MDI), also administered by the inhaled route.
Absorption
Albuterol
Healthy Subjects: Following inhaled administration of AIRSUPRA 180 mcg/160 mcg (administered as 2 inhalations of 90 mcg/80 mcg), the median tmax of albuterol was 1.00 hour (individual values ranged from 0.08 to 6.05 hours). Geometric mean Cmax was 472.2 pg/mL (individual values ranged from 38.4 to 971 pg/mL).Asthma Patients: Following increasing cumulative doses (a total dose of 1440 mcg) of albuterol (AS MDI) and an active albuterol aerosol inhaler comparator in patients with mild to moderate asthma, mean albuterol plasma concentrations (at 15 minutes after dose) increased from predose levels (44.2 and 51.4 pg/mL) to 3278.0 pg/mL and 5241.7 pg/mL with AS MDI and the comparator, respectively. Results from this study indicate the total systemic exposure of albuterol was 21% (for AUC) and 27% (for Cmax) lower for AS MDI than for the comparator.
Budesonide
Healthy Subjects: Following inhaled administration in healthy subjects of AIRSUPRA 180 mcg/160 mcg (administered as 2 inhalations of 90 mcg/80 mcg), the median tmax of budesonide was 0.33 hours (individual values ranged from 0.08 to 4.00 hours). Geometric mean Cmax was 272.8 pg/mL (individual values ranged from 51.7 to 819 pg/mL). Systemic exposure to budesonide with AIRSUPRA did not exceed that from inhaled budesonide via a dry powder inhaler comparator in healthy subjects.Distribution
Albuterol
Healthy Subjects: Following inhaled administration of AIRSUPRA 180 mcg/160 mcg (administered as 2 inhalations of 90 mcg/80 mcg), the mean apparent volume of distribution (Vz/F) of albuterol was 565.7 L.Budesonide
Healthy Subjects: Following inhaled administration of AIRSUPRA 180 mcg/160 mcg (administered as 2 inhalations of 90 mcg/80 mcg), the mean apparent volume of distribution (Vz/F) of budesonide administered in AIRSUPRA was 1002 L.Budesonide was 85-90% bound to plasma proteins. Protein binding was constant over the concentration range of 1‑100 nmol/L. Budesonide showed little or no binding to corticosteroid binding globulin. Budesonide rapidly equilibrated with red blood cells in a concentration independent manner with a blood/plasma ratio of about 0.8.
Elimination
Following inhaled single-dose administration with AIRSUPRA in healthy subjects, the mean terminal half-life of albuterol was estimated to be 7.1 hours (individual values ranged from 4.62 to 10.0 hours) and the mean terminal half-life of budesonide was estimated to be 4.1 hours (individual values ranges from 2.46 to 6.15 hours).
Metabolism
Albuterol
Information available in the published literature suggests that the primary enzyme responsible for the metabolism of albuterol in humans is SULTIA3 (sulfotransferase).Budesonide
In vitro studies with human liver homogenates have shown that budesonide is rapidly and extensively metabolized. Two major metabolites formed via cytochrome P450 (CYP) isoenzyme 3A4 (CYP3A4) catalyzed biotransformation have been isolated and identified as 16α-hydroxyprednisolone and 6β-hydroxybudesonide. The corticosteroid activity of each of these two metabolites is less than 1% of that of the parent compound. No qualitative differences between the in vitro and in vivo metabolic patterns have been detected. Negligible metabolic inactivation was observed in human lung and serum preparations.Excretion
Albuterol
The primary route of elimination of albuterol is through renal excretion of either the parent compound or the primary metabolite.Budesonide
Budesonide is excreted in urine and feces in the form of metabolites. Approximately 60% of an intravenous radiolabeled dose was recovered in the urine. No unchanged budesonide was detected in the urine.Specific Populations
Specific studies to examine the effects of age, sex, gender/ethnicity, or body weight on the pharmacokinetics of AIRSUPRA have not been conducted.
Patients with Renal or Hepatic Impairment
There are no data regarding the specific use of AIRSUPRA in patients with hepatic or renal impairment.The effect of renal impairment on the pharmacokinetics of albuterol was evaluated in 5 patients with creatinine clearance of 7 to 53 mL/min, and the results were compared with those from healthy volunteers. Renal disease had no effect on the half-life, but there was a 67% decline in albuterol clearance. Caution should be used when administering high doses of AIRSUPRA to patients with renal impairment.
Reduced liver function may affect the elimination of corticosteroids. The pharmacokinetics of budesonide were affected by compromised liver function as evidenced by a doubled systemic availability after oral ingestion. The intravenous pharmacokinetics of budesonide were, however, similar in cirrhotic patients and in healthy subjects.
Drug Interaction Studies
Inhibitors of cytochrome P450 enzymes
Ketoconazole: As a strong inhibitor of cytochrome P450 (CYP) isoenzyme 3A4 (CYP3A4), the main metabolic enzyme for corticosteroids, ketoconazole increased plasma levels of orally ingested budesonide [see Warnings and Precautions (5.11), Drug Interactions (7.1)].Cimetidine: At recommended doses, cimetidine, a nonspecific inhibitor of CYP enzymes, had a slight but clinically insignificant effect on the pharmacokinetics of oral budesonide.
-
13 NONCLINICAL TOXICOLOGY 13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - No studies of carcinogenicity, mutagenicity, or impairment of fertility were conducted with AIRSUPRA; however, available studies for ...Close
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies of carcinogenicity, mutagenicity, or impairment of fertility were conducted with AIRSUPRA; however, available studies for the individual components, albuterol and budesonide, are described below. Findings are reported based on the MRHDID for albuterol and budesonide contained in AIRSUPRA.
Albuterol
In a 2-year study in Sprague-Dawley rats, albuterol sulfate caused a dose-related increase in the incidence of benign leiomyomas of the mesovarium at and above dietary doses of 2 mg/kg/day (approximately 15 times the MRHDID for adults on a mg/m2 basis). In another study this effect was blocked by the coadministration of propranolol, a nonselective beta-adrenergic antagonist. In an 18-month study in CD-1 mice, albuterol sulfate showed no evidence of tumorigenicity at dietary doses of up to 500 mg/kg/day (approximately 1,900 times the MRHDID for adults on a mg/m2 basis). In a 22-month study in Golden Hamsters, albuterol sulfate showed no evidence of tumorigenicity at dietary doses of up to 50 mg/kg/day (approximately 250 times the MRHDID for adults on a mg/m2 basis).Albuterol sulfate was not mutagenic in the Ames test or a mutation test in yeast. Albuterol sulfate was not clastogenic in a human peripheral lymphocyte assay or in an AH1 strain mouse micronucleus assay.
Reproduction studies in rats demonstrated no evidence of impaired fertility at oral doses up to 50 mg/kg/day (approximately 380 times the MRHDID for adults on a mg/m2 basis).
Budesonide
In a 2-year study in Sprague-Dawley rats, budesonide caused a statistically significant increase in the incidence of gliomas in male rats receiving an oral dose of 50 mcg/kg/day (approximately 0.5 times the MRHDID in adults on a mcg/m2 basis). No tumorigenicity was seen in male rats at oral doses up to 25 mcg/kg/day (approximately 0.5 times the MRHDID in adults on a mcg/m2 basis) and in female rats at oral doses up to 50 mcg/kg/day (approximately 0.5 times the MRHDID doses in adults on a mcg/m2 basis). In two additional 2-year studies in male Fischer and Sprague-Dawley rats, budesonide caused no gliomas at an oral dose of 50 mcg/kg/day (approximately 0.5 times the MRHDID in adults on a mcg/m2 basis). However, in the male Sprague-Dawley rats, budesonide caused a statistically significant increase in the incidence of hepatocellular tumors at an oral dose of 50 mcg/kg/day (approximately 0.5 times the MRHDID in adults on a mcg/m2 basis). The concurrent reference corticosteroids (prednisone and triamcinolone acetonide) in these two studies showed similar findings.There was no evidence of a carcinogenic effect when budesonide was administered orally for 91 weeks to mice at doses up to 200 mcg/kg/day (equal to the MRHDID in adults on a mcg/m2 basis). Budesonide was not mutagenic or clastogenic in six different test systems: Ames Salmonella/microsome plate test, mouse micronucleus test, mouse lymphoma test, chromosome aberration test in human lymphocytes, sex-linked recessive lethal test in Drosophila melanogaster, and DNA repair analysis in rat hepatocyte culture.
Fertility and reproductive performance were unaffected in rats at subcutaneous doses up to 80 mcg/kg/day (approximately 0.8 times the MRHDID in adults on a mcg/m2 basis). At a subcutaneous dose of 20 mcg/kg/day (approximately 0.2 times the MRHDID in adults on a mcg/m2 basis), decreases in maternal body weight gain, prenatal viability, and viability of the young at birth and during lactation were observed. No such effects were noted at 5 mcg/kg/day (approximately 0.05 times the MRHDID in adults on a mcg/m2 basis).
-
14 CLINICAL STUDIES The efficacy of AIRSUPRA was evaluated in MANDALA and DENALI as described below. While patients 12 to 17 years were included in these trials, AIRSUPRA is not approved in this age group; therefore ...
The efficacy of AIRSUPRA was evaluated in MANDALA and DENALI as described below. While patients 12 to 17 years were included in these trials, AIRSUPRA is not approved in this age group; therefore, efficacy results are presented for adults only.
MANDALA
The efficacy of AIRSUPRA 180 mcg/160 mcg was evaluated in patients 12 years of age and older with moderate to severe asthma in MANDALA (NCT03769090), a randomized, double-blind, multicenter study. MANDALA was a variable length exacerbation study with at least 24 weeks duration.Patients 12 years of age and older were randomized (1:1:1) and received at least one dose of AIRSUPRA (AS-BD MDI 180 mcg/160 mcg), albuterol/budesonide (AS-BD MDI 180 mcg/80 mcg) or albuterol (AS MDI 180 mcg) as needed. Patients were required to be receiving medium to high dose ICS or low to high dose ICS/long-acting beta2-adrenergic agonists (LABA), with or without another controller medicine as maintenance therapy. All patients continued their own maintenance therapy throughout the trial. MANDALA was conducted in symptomatic patients with moderate to severe asthma, a history of at least 1 severe asthma exacerbation in the year prior to screening, pre-bronchodilator FEV1 40 to < 90% of predicted normal (for patients aged 12 to < 18 years old, ≥ 60%) and a confirmed reversibility to albuterol.
The adult efficacy population (n=2940) in MANDALA had a mean age of 52 years (range: 18-84 years), with 66% female and 82% Caucasian, and 25% of Hispanic or Latino ethnicity. The median duration of asthma was 21 years (range 1 to 80 years) since the first diagnosis of asthma. The mean pre-bronchodilator percent predicted FEV1 was 63% (range 33% - 101%).
The overall patterns of use of AIRSUPRA and AS MDI 180 mcg were similar during the randomized treatment period.
The primary efficacy endpoint was the time to first severe asthma exacerbation (defined as worsening or onset of asthma symptoms that required systemic corticosteroids for at least 3 days or an emergency room visit that led to the use of systemic corticosteroids for at least 3 days or a hospitalization for at least 24 hours due to asthma).
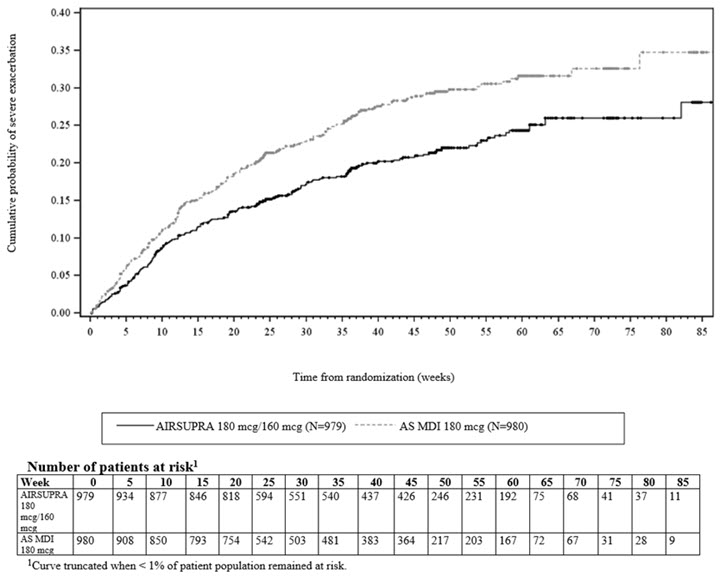
Efficacy results are shown for only the approved adult population. Treatment with AIRSUPRA, compared with AS MDI 180 mcg, demonstrated statistically significant reductions in the risk of severe asthma exacerbations among adult patients, as assessed by the time to first severe asthma exacerbation as described in Figure 1. Compared with AS MDI 180 mcg, patients receiving AIRSUPRA experienced a statistically significant 28% reduction (Hazard Ratio [HR] 0.72; 95% CI: 0.60, 0.86) in the risk of a severe asthma exacerbation (p<0.001).
Figure 1. Kaplan-Meier Cumulative Incidence Curve for Time to First Exacerbation1
There were reductions in severe asthma exacerbation risk regardless of exacerbation history (1 or > 1 in the past year), baseline lung function, and asthma severity (defined by background maintenance therapy) compared with AS MDI 180 mcg.
Treatment with AIRSUPRA 180 mcg/160 mcg statistically significantly reduced the annual rate of severe asthma exacerbations by 24% compared with AS MDI 180 mcg as shown in Table 2.
Table 2 Annualized Rate of Severe Exacerbations in Patients with Moderate to Severe Asthma Analysis Group
n
Number
of Severe ExacerbationsAnnualized
RateRate Ratio vs
AS MDI
(95% CI)P-value
AIRSUPRA
180 mcg/160 mcg≥ 18 years
979
324
0.46
0.76 (0.62, 0.93)
p=0.008
AS MDI 180 mcg
≥ 18 years
980
403
0.60
The annualized total systemic corticosteroid (SCS) dose due to asthma was calculated for each patient during the study period. For patients treated with AIRSUPRA 180 mcg/160 mcg the annualized total SCS dose when compared with AS MDI 180 mcg was statistically significantly different, with a reduction in mean annualized dose of 40 mg per patient [AIRSUPRA (87 mg) vs AS MDI 180 (127 mg)].
Asthma control was assessed using the Asthma Control Questionnaire (ACQ-5) responder analysis. Responders were defined as those patients with an improvement of 0.5 or more in overall ACQ-5 score at Week 24 compared to baseline. The percentage of ACQ-5 adult responders at Week 24 for AIRSUPRA 180 mcg/160 mcg was 67% compared with 63% for AS MDI 180 mcg (odds ratio 1.21; 95% CI: 1.01, 1.46).
Asthma quality of life was assessed using the Asthma Quality of Life Questionnaire in patients 12 years of age and older (AQLQ+12). Responders were defined as those with an improvement of 0.5 or more in AQLQ score at Week 24 compared to baseline. The percentage of adult responders at Week 24 for AIRSUPRA 180 mcg/160 mcg was 52% (odds ratio 1.22; 95% CI: 1.01, 1.47) compared with 47% for AS MDI 180 mcg.
DENALI
The efficacy of AIRSUPRA on lung function was evaluated in patients 12 years of age and older with mild to moderate asthma who were previously treated with as-needed short-acting beta2-agonists (SABA) alone or with low-dose ICS maintenance therapy plus as-needed SABA. DENALI (NCT03847896) was a double-blind, active-comparator and placebo-controlled lung function study conducted over 12 weeks. Patients were randomized 1:1:1:1:1 to receive AIRSUPRA (AS-BD MDI 180 mcg/160 mcg), AS-BD MDI 180 mcg/80 mcg, BD MDI 160 mcg, AS MDI 180 mcg, or Placebo MDI, all administered 4 times daily. Only the results for the approved recommended dose of AIRSUPRA are described below.The population 18 years of age and older (n=964) had a mean age of 50 years (range: 18-90 years) with 63% female and 89% Caucasian, and 28% of Hispanic or Latino ethnicity.
Efficacy results are presented for only the approved adult population. The onset of bronchodilation (as defined by a ≥ 15% increase in FEV1 post-dose within 30 minutes on Day 1) was observed in 51% of patients treated with AIRSUPRA 180 mcg/160 mcg and 43% of patients treated with AS MDI 180 mcg. Following a single dose on Day 1, the median time to onset and mean duration of bronchodilation were 7.5 and 186.9 minutes with AIRSUPRA 180 mcg/160 mcg and 10.0 and 167.9 minutes with AS MDI 180 mcg, respectively.
Close -
16 HOW SUPPLIED/STORAGE AND HANDLING AIRSUPRA Inhalation Aerosol is supplied as a pressurized aluminum canister with an attached dose indicator and a white plastic actuator, with a frosted clear plastic dust cap tethered to it. Each ...
AIRSUPRA Inhalation Aerosol is supplied as a pressurized aluminum canister with an attached dose indicator and a white plastic actuator, with a frosted clear plastic dust cap tethered to it. Each canister is packaged in a foil overwrap pouch with a desiccant sachet and placed into a carton. Each carton contains one canister and a Patient Information leaflet. Each 120-inhalation canister has a net fill weight of 10.7 grams.
AIRSUPRA is available in the following package size as presented in Table 3:
Table 3 Package Information for AIRSUPRA Package Size
Strength
albuterol/budesonideNumber of Inhalations per Canister
NDC
AIRSUPRA
90 mcg/80 mcg
120
0310-9080-12
The AIRSUPRA canister should only be used with the AIRSUPRA actuator, and the AIRSUPRA actuator should not be used with any other inhalation drug product.
Counter
The correct amount of medication in each inhalation cannot be ensured after the labeled number of inhalations from the canister have been used, when the dose indicator pointer reaches zero, even though the canister may not feel completely empty. AIRSUPRA should be discarded when the dose indicator pointer reaches zero, or 12 months after removal of the foil pouch, whichever comes first. Never immerse the canister in water to determine the amount remaining in the canister (“float test”).
Storage
Store at controlled room temperature 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP]. Keep in a dry place away from heat and sunlight.
For best results, the canister should be at room temperature before use. Shake well before using. Keep out of reach of children.
Contents under pressure. Do not puncture. Do not use or store near heat or open flame. Exposure to temperatures above 49°C (120°F) may cause bursting. Never throw canister into fire or incinerator. Avoid spraying in eyes.
Close -
17 PATIENT COUNSELING INFORMATION Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Deterioration of Asthma - Inform patients to seek medical attention immediately if ...
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Deterioration of Asthma
Inform patients to seek medical attention immediately if treatment with AIRSUPRA becomes less effective for symptomatic relief, and/or symptoms become worse [see Warnings and Precautions (5.1)].
Paradoxical Bronchospasm
Instruct patients to discontinue AIRSUPRA and contact their healthcare provider right away if they develop paradoxical bronchospasm [see Warnings and Precautions (5.2)].
Do Not Exceed Recommended Dosage
Instruct patients concerning the recommended dosage of AIRSUPRA and not to exceed 6 doses (12 inhalations) in a 24-hour period [see Dosage and Administration (2.1), Warnings and Precautions (5.4)].
Hypersensitivity Reactions, Including Anaphylaxis
Advise patients to contact their healthcare provider and discontinue AIRSUPRA if hypersensitivity reactions (e.g., anaphylaxis, rash, urticaria, angioedema, and bronchospasm) occur with AIRSUPRA use [Warnings and Precautions (5.5)].
Priming and Cleaning Inhaler
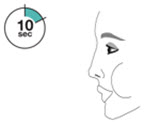
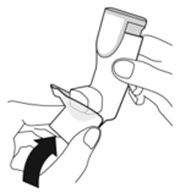
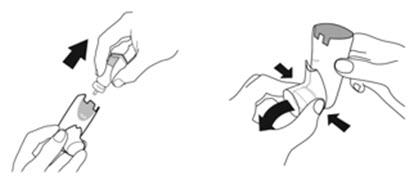
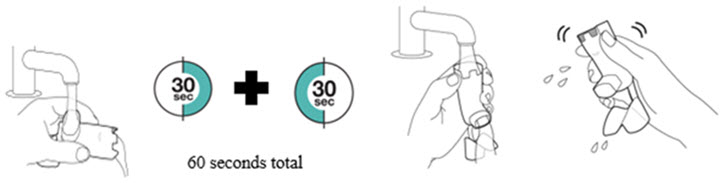
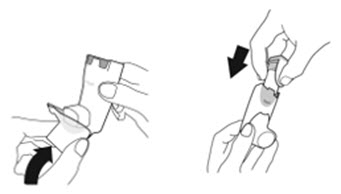
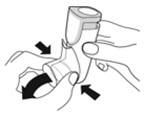
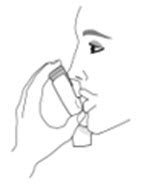
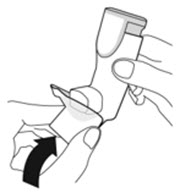
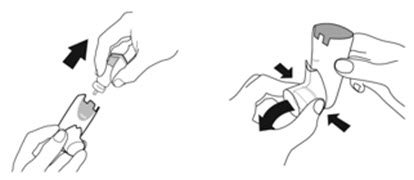
Instruct patients to prime the inhaler before using for the first time, when the inhaler has not been used for more than 7 days, is dropped, or after cleaning and to shake well before each spray.
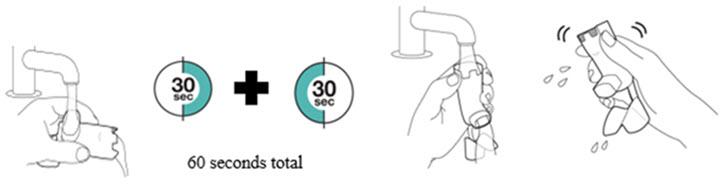
To ensure proper dosing and to prevent actuator mouthpiece blockage, instruct patients how to clean the actuator [see Dosage and Administration (2.1) and Instructions for Use].
Use with Other Short-Acting Bronchodilators
Advise patients concerning the appropriate use of other short-acting bronchodilators while using AIRSUPRA [see Drug Interactions (7.2)].
Immunosuppression and Risk of Infections
Warn patients who are on immunosuppressant doses of corticosteroids to avoid exposure to chickenpox or measles and, if exposed, to consult their physician without delay. Patients should be informed of potential worsening of existing tuberculosis, fungal, bacterial, viral, or parasitic infections, or ocular herpes simplex [see Warnings and Precautions (5.8)].
Oropharyngeal Candidiasis
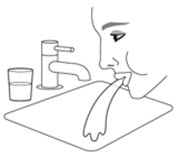
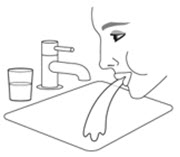
Patients should be advised that localized infections with Candida albicans occurred in the mouth and pharynx in some patients. Advise patients to rinse the mouth with water, if available, without swallowing after AIRSUPRA inhalation to help reduce the risk of thrush [see Warnings and Precautions (5.9)].
Hypercorticism and Adrenal Suppression
Advise patients that AIRSUPRA may cause systemic corticosteroid effects of hypercorticism and adrenal suppression [see Warnings and Precautions (5.10)].
Reduction in Bone Mineral Density
Advise patients who are at an increased risk for decreased BMD that the use of AIRSUPRA may pose an additional risk [see Warnings and Precautions (5.11)].
Glaucoma and Cataracts
Inform patients that long-term use of AIRSUPRA may increase the risk of increased intraocular pressure, glaucoma, and cataracts; consider regular eye examinations [see Warnings and Precautions (5.12)].
Pregnancy
Advise pregnant women that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to asthma medications during pregnancy and to contact the MotherToBaby Pregnancy Studies at 1-877-311-8972 or visit https://mothertobaby.org/ongoing-study/asthma/.
Manufactured for: AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850
Manufactured by: AstraZeneca Dunkerque Production (AZDP),
Dunkerque, FranceAIRSUPRA is a registered trademark of the AstraZeneca group of companies.
Close
©AstraZeneca 2024 -
Patient Package Insert PATIENT INFORMATION - AIRSUPRA® (ayr soo' prah) (albuterol and budesonide) inhalation aerosol, for oral inhalation use - What is AIRSUPRA? AIRSUPRA combines 2 medicines, a ...
PATIENT INFORMATION
AIRSUPRA® (ayr soo' prah)
(albuterol and budesonide)
inhalation aerosol, for oral inhalation useWhat is AIRSUPRA?
AIRSUPRA combines 2 medicines, a short/rapid-acting beta2-adrenergic agonist (SABA) medicine (albuterol sulfate) and an inhaled corticosteroid (ICS) medicine (budesonide), in 1 inhaler, delivered as a propelled spray.
- •
- SABA medicines such as albuterol help to relax the smooth muscles of the airways, causing the airways to widen, leading to easier breathing.
- •
- ICS medicines such as budesonide help to decrease inflammation in the lungs. Inflammation in the lungs can lead to breathing difficulties.
- •
- AIRSUPRA is a prescription medicine used:
- •
- as needed as a rescue inhaler to treat or prevent symptoms of asthma (tightening of the airways, wheezing, cough and shortness of breath).
- •
- to help prevent sudden severe breathing problems (asthma attacks).
- •
- AIRSUPRA is used as 2 inhalations (puffs) as needed. Do not use more than 12 inhalations (puffs) in a 24-hour period.
- •
- AIRSUPRA is not to be used as maintenance treatment for asthma. If you are currently taking medicine long-term to maintain control of asthma symptoms, you should continue to take that medicine as directed by your healthcare provider.
- •
- It is not known if AIRSUPRA is safe and effective in children.
Do not use AIRSUPRA if you:
- •
- are allergic to albuterol sulfate, budesonide, or any of the other ingredients in AIRSUPRA. See the end of this Patient Information leaflet for a complete list of ingredients in AIRSUPRA.
Before using AIRSUPRA, tell your healthcare provider about all your medical conditions, including if you:
- •
- have heart problems.
- •
- have high blood pressure (hypertension).
- •
- have convulsions (seizures).
- •
- have thyroid problems.
- •
- have diabetes.
- •
- have low potassium levels in your blood.
- •
- have an immune system problem.
- •
- have or had tuberculosis of your respiratory tract.
- •
- have had any type of viral, bacterial, parasitic, fungal infection, or herpes simplex infection of the eye (ocular herpes simplex) that has not been treated.
- •
- have been exposed to chickenpox or measles or you have not received vaccines against these diseases.
- •
- have or are at risk for weak bones. You are at risk for weak bones if you:
- •
- are inactive for a long period of time
- •
- have a family history of osteoporosis
- •
- are a woman going through menopause (“the change of life”) or are past menopause
- •
- smoke or use tobacco
- •
- are elderly
- •
- do not eat well (poor nutrition)
- •
- use medicines that cause bone thinning (such as seizure medicines or corticosteroids) for a long time
- •
- have eye problems such as increased pressure in the eye, glaucoma, or cataracts.
- •
- have liver problems.
- •
- are pregnant or plan to become pregnant. It is not known if AIRSUPRA may harm your unborn baby.
- •
- Pregnancy Registry. There is a pregnancy registry for women who use asthma medicines during pregnancy. If you become pregnant, contact the MotherToBaby Pregnancy Studies conducted by the Organization of Teratology Information Specialists at 1-877-311-8972 or visit https://mothertobaby.org/ongoing-study/asthma/.
- •
- are breastfeeding. It is not known if the medicines in AIRSUPRA pass into your breast milk and if they can harm your baby. You and your healthcare provider should decide if you will take AIRSUPRA while breastfeeding.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. AIRSUPRA and certain other medicines may affect each other causing side effects.
Especially, tell your healthcare provider if you take:
- •
- medicines known as strong CYP3A4 inhibitors
- •
- other inhaled medicines or asthma medicines
- •
- beta blockers
- •
- diuretics
- •
- digoxin
- •
- monoamine oxidase inhibitors
- •
- tricyclic antidepressants
Ask your healthcare provider or pharmacist for a list of these medicines, if you are not sure.
Know the medicines that you take. Keep a list of them to show your healthcare provider and pharmacist each time you get a new medicine.
How should I use AIRSUPRA?
Read the step-by-step instructions for using AIRSUPRA at the end of this Patient Information leaflet.- •
- Before using AIRSUPRA, make sure your healthcare provider has taught you how to use the inhaler and that you understand how to use it correctly.
- •
- Use AIRSUPRA exactly as your healthcare provider tells you to use it. Do not use AIRSUPRA more often than prescribed (no more than 12 puffs which equals 6 doses) within a 24-hour period.
- •
- Do not change or stop other inhaled medicines or asthma medicines (oral or inhaled) without first talking to your healthcare provider.
- •
-
Call your healthcare provider or get emergency medical care right away if:
- •
- your breathing problems get worse.
- •
- you need to use AIRSUPRA more often than usual.
- •
- AIRSUPRA does not work as well to relieve your asthma.
What are the possible side effects of AIRSUPRA?
AIRSUPRA can cause serious side effects, including:- •
-
worsening trouble breathing, coughing, and wheezing (paradoxical bronchospasm). If this happens, stop using AIRSUPRA and call your healthcare provider or get emergency medical care right away. Paradoxical bronchospasm is more likely to happen with your first use of a new canister of medicine.
- •
-
heart problems, including faster heart rate and higher blood pressure.
- •
-
possible death in people who use too much AIRSUPRA.
- •
-
serious allergic reactions. Tell your healthcare provider or get emergency medical care right away if you have:
- •
- skin rash, redness, or swelling
- •
- severe itching
- •
- swelling of the face, mouth, and tongue
- •
- trouble breathing or swallowing
- •
- chest pain
- •
-
changes in laboratory blood levels. Low levels of potassium (hypokalemia) may cause abnormal heart rhythms. Your healthcare provider may do blood tests to check your potassium levels during treatment with AIRSUPRA.
- •
- weakened immune system and increased chance of getting infections. Tell your healthcare provider about any signs and symptoms of infection including:
- •
- fever
- •
- pain
- •
- aches
- •
- chills
- •
- feeling tired
- •
- nausea and vomiting
- •
-
fungal infection in your mouth and throat (thrush). This is a common side effect. Rinse your mouth with water, if available, without swallowing after using AIRSUPRA to help reduce your chance of getting thrush.
- •
-
reduced adrenal function (adrenal insufficiency). Adrenal insufficiency is a condition in which the adrenal glands do not make enough steroid hormones. This can happen when you start taking a medicine containing an ICS (such as AIRSUPRA). When your body is under stress from fever, trauma (such as a car accident), infection or surgery, adrenal insufficiency can get worse.
Symptoms of adrenal insufficiency include:
- •
- feeling tired
- •
- lack of energy
- •
- weakness
- •
- nausea and vomiting
- •
- low blood pressure
- •
-
bone thinning or weakness (osteoporosis). Your healthcare provider should check you for this during treatment with AIRSUPRA.
- •
-
eye problems, including glaucoma and cataracts. You should have regular eye exams while using AIRSUPRA.
Call your healthcare provider or get medical help right away if you have symptoms of any of the serious side effects listed above.
Common side effects of AIRSUPRA include:-
- •
- headache
- •
- cough
- •
- hoarseness
Tell your healthcare provider about any side effect that bothers you or that does not go away.
These are not all the side effects of AIRSUPRA. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to AstraZeneca at 1-800-236-9933.
How should I store AIRSUPRA?
- •
- Store AIRSUPRA at room temperature between 68ºF to 77ºF (20ºC to 25ºC). Keep in a dry place away from heat and sunlight.
- •
- Store AIRSUPRA in the unopened foil pouch and only open when ready for use.
- •
- Do not put a hole in the AIRSUPRA canister.
- •
- Do not use or store AIRSUPRA near heat or a flame. Temperatures above 120ºF (49ºC) may cause the canister to burst.
- •
- Do not throw the AIRSUPRA canister into a fire or an incinerator.
- •
- Throw away AIRSUPRA 12 months after you open the foil pouch or when the puff indicator reaches zero “0”; whichever comes first.
Keep AIRSUPRA and all medicines out of the reach of children.
General information about the safe and effective use of AIRSUPRA
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use AIRSUPRA for a condition for which it was not prescribed. Do not give your AIRSUPRA to other people, even if they have the same condition that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about AIRSUPRA that is written for healthcare professionals.What are the ingredients in AIRSUPRA?
Active ingredients: micronized albuterol sulfate and micronized budesonide.
Inactive ingredients: hydrofluoroalkane (HFA 134a) and porous particles 1,2-Distearoyl-sn-glycero-3-phosphocholine (DSPC) and calcium chloride.
AIRSUPRA is a registered trademark of the AstraZeneca group of companies ©AstraZeneca 2024Manufactured for: AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850; Manufactured by: AstraZeneca Dunkerque Production (AZDP), Dunkerque, France
For more information, go to www.AIRSUPRA.com or call 1-800-236-9933.
This Patient Information has been approved by the U.S. Food and Drug Administration. Issued: 03/2024
Close -
Instructions for Use AIRSUPRA® (ayr soo' prah) (albuterol and budesonide) inhalation aerosol, for oral inhalation use - 120-inhalation canister - Read this Instructions for Use before you start using AIRSUPRA and ...
AIRSUPRA® (ayr soo' prah)
(albuterol and budesonide)
inhalation aerosol, for oral inhalation use
120-inhalation canisterRead this Instructions for Use before you start using AIRSUPRA and each time you get a new refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
Important information
- •
- For oral inhalation use only.
- •
- Use AIRSUPRA exactly as your healthcare provider tells you to.
- •
- Before you use AIRSUPRA for the first time, you will need to prime it.
- •
- Rinse the white actuator 1-time each week.
- •
- 2 puffs of medicine is 1-dose. Do not use more than 6 doses (12 puffs) in a 24-hour period.
- •
- Rinse your mouth with water, if available, after using AIRSUPRA to decrease the chance of getting a fungal infection (thrush) in your mouth and throat.
Parts of your AIRSUPRA inhaler
Reading the puff indicator
- •
- The puff indicator will count down by 1 each time you spray a puff of medicine.
Do not try to take a puff of medicine when the pointer is at 0 because you will not receive a full dose.
Before using your AIRSUPRA inhaler
1. Write the Use by date
Write the Use By Date (12 months after you open the foil pouch) on the actuator label using permanent ink.
Write the Use By Date on the actuator label.
How to use your inhaler - Inhaling your medicine
1. Check
Take the cover off the mouthpiece. Check the mouthpiece for foreign objects and remove objects before use.
Check inside mouthpiece.
Weekly rinse - Rinsing your actuator 1 time each week
- •
- Rinse the white actuator 1-time each week so that the medicine does not build up and block the spray through the mouthpiece.
- •
- Do not allow the canister to get wet.
- •
- Re-prime your AIRSUPRA inhaler after rinsing.
Emergency inhaler use when wet
If you need to use the inhaler before it is dry:
- •
- shake off as much excess water as you can
- •
- insert the canister into the actuator
- •
- shake and spray 2-test puffs into the air away from your face
- •
- take the prescribed dose
- •
- rinse the actuator again following the Weekly Rinse steps.
Storing your inhaler
- •
- Store your inhaler at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- Do not store in a humid environment, such as a bathroom.
- •
- Keep your inhaler and all medicines out of the reach of children.
Throwing away your AIRSUPRA inhaler
Throw away your AIRSUPRA inhaler in the household trash:
- •
- when the puff indicator reaches zero “0” or 12 months after you open the foil pouch, whichever comes first.
Do not reuse or use the actuator with medicine canisters from other inhalers.
Do not puncture or throw the canister into a fire or incinerator.Ordering a new AIRSUPRA inhaler
- •
- Order a new AIRSUPRA inhaler when the pointer on the puff indicator is in the yellow zone.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Issued: March 2024
Close -
Instructions for Use AIRSUPRA® (ayr soo' prah) (albuterol and budesonide) inhalation aerosol, for oral inhalation use - 28-inhalation canister - Read this Instructions for Use before you start using AIRSUPRA and ...
AIRSUPRA® (ayr soo' prah)
(albuterol and budesonide)
inhalation aerosol, for oral inhalation use
28-inhalation canisterRead this Instructions for Use before you start using AIRSUPRA and each time you get a new refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
Important information
- •
- For oral inhalation use only.
- •
- Use AIRSUPRA exactly as your healthcare provider tells you to.
- •
- Before you use AIRSUPRA for the first time, you will need to prime it.
- •
- Rinse the white actuator 1-time each week.
- •
- 2 puffs of medicine is 1-dose. Do not use more than 6 doses (12 puffs) in a 24-hour period.
- •
- Rinse your mouth with water, if available, after using AIRSUPRA to decrease the chance of getting a fungal infection (thrush) in your mouth and throat.
Parts of your AIRSUPRA inhaler
Reading the puff indicator
- •
- The puff indicator will count down by 1 each time you spray a puff of medicine.
Do not try to take a puff of medicine when the pointer is at 0 because you will not receive a full dose.
Before using your AIRSUPRA inhaler
1. Write the Use by date
Write the Use By Date (6 months after you open the foil pouch) on the actuator label using permanent ink.
Write the Use By Date on the actuator label.
How to use your inhaler - Inhaling your medicine
1. Check
Take the cover off the mouthpiece. Check the mouthpiece for foreign objects and remove objects before use.
Check inside mouthpiece.
Weekly rinse - Rinsing your actuator 1 time each week
- •
- Rinse the white actuator 1-time each week so that the medicine does not build up and block the spray through the mouthpiece.
- •
- Do not allow the canister to get wet.
- •
- Re-prime your AIRSUPRA inhaler after rinsing.
Emergency inhaler use when wet
If you need to use the inhaler before it is dry:
- •
- shake off as much excess water as you can
- •
- insert the canister into the actuator
- •
- shake and spray 2-test puffs into the air away from your face
- •
- take the prescribed dose
- •
- rinse the actuator again following the Weekly Rinse steps.
Storing your inhaler
- •
- Store your inhaler at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- Do not store in a humid environment, such as a bathroom.
- •
- Keep your inhaler and all medicines out of the reach of children.
Throwing away your AIRSUPRA inhaler
Throw away your AIRSUPRA inhaler in the household trash:
- •
- when the puff indicator reaches zero “0” or 6 months after you open the foil pouch, whichever comes first.
Do not reuse or use the actuator with medicine canisters from other inhalers.
Do not puncture or throw the canister into a fire or incinerator.Ordering a new AIRSUPRA inhaler
- •
- Order a new AIRSUPRA inhaler when the pointer on the puff indicator is in the yellow zone.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Issued: March 2024
Close -
Package/Label Display Panel NDC 0310-9080-12 Rx only - AIRSUPRA® (albuterol and budesonide) inhalation aerosol - 90 mcg/80 mcg - Per inhalation - For Oral ...
NDC 0310-9080-12 Rx only
AIRSUPRA®
(albuterol and budesonide)
inhalation aerosol
90 mcg/80 mcg
Per inhalation
For Oral Inhalation only
Shake inhaler well before using
120 inhalations
AstraZeneca
AIRSUPRA®
(albuterol and budesonide)
inhalation aerosol
90 mcg/80 mcg
Per inhalation
Close -
INGREDIENTS AND APPEARANCEProduct Information
AIRSUPRA albuterol sulfate and budesonide aerosol, metered Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0310-9080 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALBUTEROL SULFATE (UNII: 021SEF3731) (ALBUTEROL - UNII:QF8SVZ843E) ALBUTEROL 90 ug BUDESONIDE (UNII: Q3OKS62Q6X) (BUDESONIDE - UNII:Q3OKS62Q6X) BUDESONIDE 80 ug Inactive Ingredients Ingredient Name Strength 1,2-DISTEAROYL-SN-GLYCERO-3-PHOSPHOCHOLINE (UNII: 043IPI2M0K) CALCIUM CHLORIDE (UNII: M4I0D6VV5M) NORFLURANE (UNII: DH9E53K1Y8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0310-9080-12 1 in 1 CARTON 10/02/2023 1 120 in 1 INHALER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:0310-9080-28 1 in 1 CARTON 10/02/2023 2 28 in 1 INHALER; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA214070 10/02/2023 Labeler - AstraZeneca Pharmaceuticals LP (054743190)
CloseRegistrant - AstraZeneca PLC (230790719)
Find additional resources
(also available in the left menu)Safety
Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
AIRSUPRA- albuterol sulfate and budesonide aerosol, metered
Number of versions: 2
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Apr 22, 2024 | 9 (current) | download |
| Oct 2, 2023 | 3 | download |
RxNorm
AIRSUPRA- albuterol sulfate and budesonide aerosol, metered
| RxCUI | RxNorm NAME | RxTTY | |
|---|---|---|---|
| 1 | 2665906 | albuterol 90 MCG/INHAL / budesonide 80 MCG/INHAL Metered Dose Inhaler, 120 Actuations | PSN |
| 2 | 2665906 | 120 ACTUAT albuterol 0.09 MG/ACTUAT / budesonide 0.08 MG/ACTUAT Metered Dose Inhaler | SCD |
| 3 | 2665906 | albuterol 90 MCG/INHAL / budesonide 80 MCG/INHAL Metered Dose Inhaler, 120 Actuations | SY |
| 4 | 2665906 | albuterol sulfate 108 MCG/INHAL / budesonide 80 MCG/INHAL Metered Dose Inhaler, 120 Actuations | SY |
| 5 | 2665912 | AIRSUPRA 90/80 MCG/INHAL Metered Dose Inhaler, 120 Actuations | PSN |
| 6 | 2665912 | 120 ACTUAT albuterol 0.09 MG/ACTUAT / budesonide 0.08 MG/ACTUAT Metered Dose Inhaler [Airsupra] | SBD |
| 7 | 2665912 | Airsupra (albuterol 90 MCG/INHAL / budesonide 80 MCG/INHAL) Metered Dose Inhaler, 120 Actuations | SY |
| 8 | 2665912 | Airsupra (albuterol sulfate 108 MCG/INHAL / budesonide 80 MCG/INHAL) Metered Dose Inhaler, 120 Actuations | SY |
| 9 | 2678391 | albuterol 90 MCG/INHAL / budesonide 80 MCG/INHAL Metered Dose Inhaler, 28 Actuations | PSN |
| 10 | 2678391 | 28 ACTUAT albuterol 0.09 MG/ACTUAT / budesonide 0.08 MG/ACTUAT Metered Dose Inhaler | SCD |
| 11 | 2678391 | albuterol sulfate 108 MCG/ACTUAT / budesonide 80 MCG/ACTUAT Metered Dose Inhaler, 28 Actuations | SY |
| 12 | 2678392 | AIRSUPRA 90/80 MCG/INHAL Metered Dose Inhaler, 28 Actuations | PSN |
| 13 | 2678392 | 28 ACTUAT albuterol 0.09 MG/ACTUAT / budesonide 0.08 MG/ACTUAT Metered Dose Inhaler [Airsupra] | SBD |
| 14 | 2678392 | Airsupra (albuterol 90 MCG/ACTUAT / budesonide 80 MCG/ACTUAT) Metered Dose Inhaler, 28 Actuations | SY |
| 15 | 2678392 | Airsupra (albuterol sulfate 108 MCG/ACTUAT / budesonide 80 MCG/ACTUAT) Metered Dose Inhaler, 28 Actuations | SY |
Get Label RSS Feed for this Drug
AIRSUPRA- albuterol sulfate and budesonide aerosol, metered
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=e60aa9f9-5139-44df-b886-ebddc3c7eb13
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
AIRSUPRA- albuterol sulfate and budesonide aerosol, metered
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 0310-9080-12 |
| 2 | 0310-9080-28 |