Label: BEPOTASTINE BESILATE solution/ drops
- NDC Code(s): 82260-630-05, 82260-630-10
- Packager: Bausch & Lomb Americas Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated August 20, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Bepotastine Besilate Ophthalmic Solution safely and effectively. See full prescribing information for Bepotastine Besilate Ophthalmic ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEBepotastine Besilate Ophthalmic Solution, 1.5% is a histamine H - 1receptor antagonist indicated for the treatment of itching associated with signs and symptoms of allergic conjunctivitis ...
-
2 DOSAGE AND ADMINISTRATIONInstill one drop of Bepotastine Besilate Ophthalmic Solution into the affected eye(s) twice a day. Remove contact lenses prior to instillation of Bepotastine Besilate Ophthalmic Solution.
-
3 DOSAGE FORMS AND STRENGTHSOphthalmic solution containing bepotastine besilate 15 mg/mL (1.5%).
-
4 CONTRAINDICATIONSBepotastine Besilate Ophthalmic Solution is contraindicated in patients with a history of hypersensitivity reactions to bepotastine or any of the other ingredients - [see - Adverse Reactions ...
-
5 WARNINGS AND PRECAUTIONS5.1 Contamination of Tip and Solution - To minimize contaminating the dropper tip and solution, advise the patient not to touch the eyelids or surrounding areas with the dropper tip of the bottle ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no available human data for the use of Bepotastine Besilate Ophthalmic Solution during pregnancy to inform any drug-associated risks. Oral administration ...
-
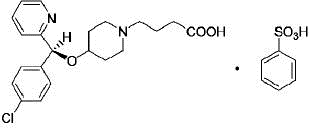
11 DESCRIPTIONBepotastine Besilate Ophthalmic Solution, 1.5% is a sterile, topically administered drug for ophthalmic use. Each mL of Bepotastine Besilate Ophthalmic Solution contains 15 mg bepotastine ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Bepotastine is a topically active, direct H1-receptor antagonist and an inhibitor of the release of histamine from mast cells. 12.3 Pharmacokinetics - Absorption:The ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term dietary studies in mice and rats were conducted to evaluate the carcinogenic potential of bepotastine ...
-
14 CLINICAL STUDIESClinical efficacy was evaluated in two conjunctival allergen challenge (CAC) studies (237 patients). Bepotastine Besilate Ophthalmic Solution, 1.5% was more effective than its vehicle for ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGBepotastine Besilate Ophthalmic Solution, 1.5% is supplied in a white low density polyethylene bottle with a sterile linear low density polyethylene controlled dropper tip and a white ...
-
17 PATIENT COUNSELING INFORMATIONSterility of Dropper Tip - Advise patients not to touch the dropper tip to any surface, as this may contaminate the solution and to keep the bottle tightly closed when not in use. Distributed ...
-
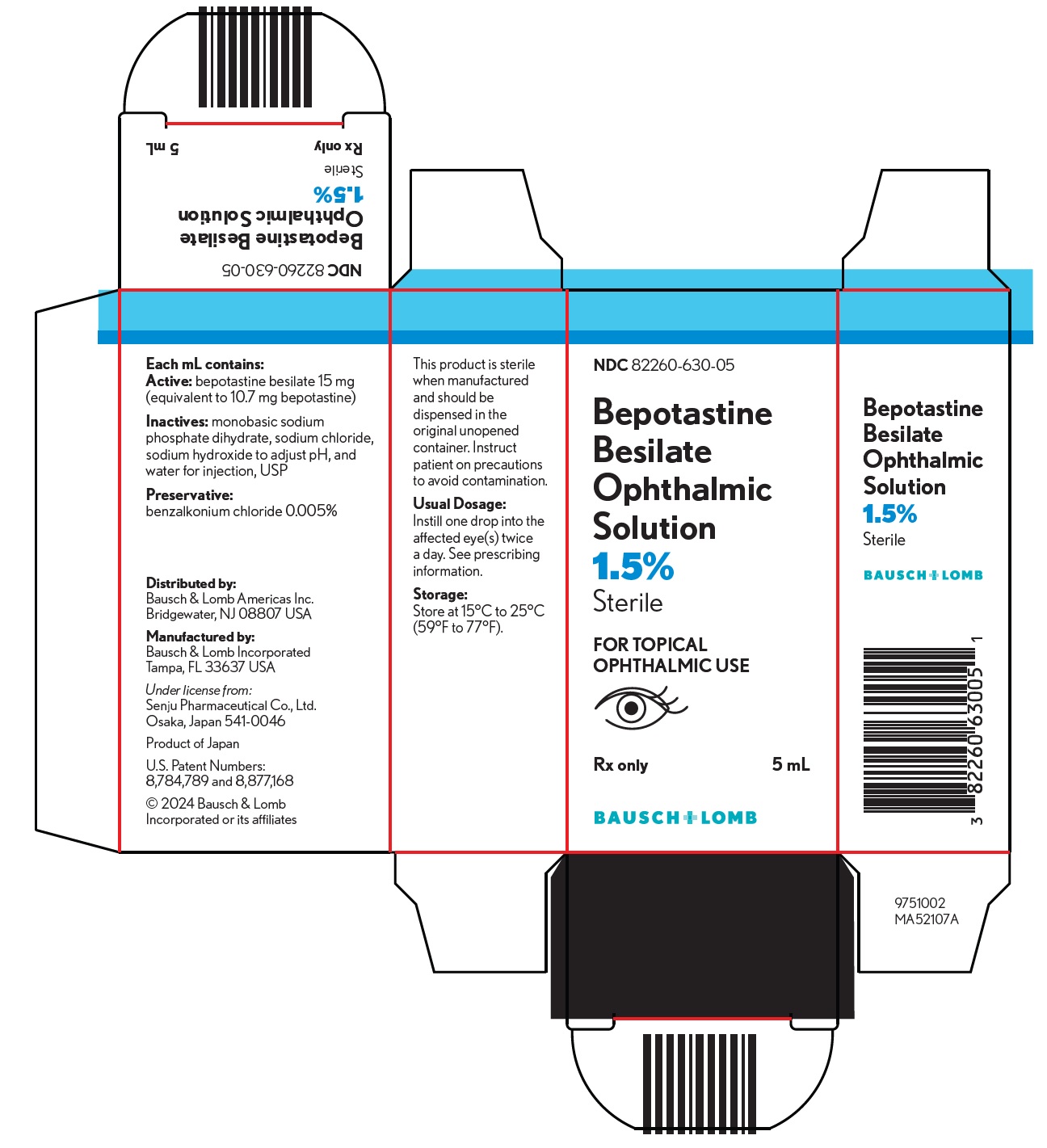
PACKAGE/LABEL PRINCIPAL DISPLAY PANELNDC82260-630-05 - Bepotastine - Besilate - Ophthalmic - Solution - 1.5% Sterile - FOR TOPICAL - OPHTHALMIC USE - (eye image) Rx only5 mL - BAUSCH + LOMB - 9751002 - MA52107 A
-
INGREDIENTS AND APPEARANCEProduct Information