Label: BRIMONIDINE TARTRATE AND TIMOLOL MALEATE- brimonidine tartrate, timolol maleate solution/ drops
- NDC Code(s): 82182-455-05, 82182-455-10, 82182-455-15
- Packager: Pacific Pharma, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application Authorized Generic
Drug Label Information
Updated July 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BRIMONIDINE TARTRATE and TIMOLOL MALEATE OPHTHALMIC SOLUTION safely and effectively. See full prescribing information for ...
-
Table of ContentsTable of Contents
-
1
INDICATIONS AND USAGE
Brimonidine tartrate and timolol maleate ophthalmic solution 0.2%/0.5% is an alpha-adrenergic receptor agonist with a beta-adrenergic receptor inhibitor indicated for the reduction of elevated ...
-
2
DOSAGE AND ADMINISTRATION
The recommended dose is one drop of brimonidine tartrate and timolol maleate ophthalmic solution in the affected eye(s) twice daily approximately 12 hours apart. If more than one topical ...
-
3
DOSAGE FORMS AND STRENGTHS
Solution containing 2 mg/mL brimonidine tartrate and 5 mg/mL timolol (6.8 mg/mL timolol maleate).
-
4
CONTRAINDICATIONS
4.1 - Reactive Airway Disease Including Asthma, COPD - Brimonidine tartrate and timolol maleate ophthalmic solution is contraindicated in patients with reactive airway disease including ...
-
5
WARNINGS AND PRECAUTIONS
5.1 - Potential for Severe Respiratory or Cardiac Reactions - Brimonidine tartrate and timolol maleate ophthalmic solution contains timolol maleate; and although administered topically ...
-
6
ADVERSE REACTIONS
6.1 - Clinical Studies Experience - Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be ...
-
7
DRUG INTERACTIONS
7.1 - Antihypertensives/Cardiac Glycosides - Because brimonidine tartrate and timolol maleate ophthalmic solution may reduce blood pressure, caution in using drugs such as ...
-
8
USE IN SPECIFIC POPULATIONS
8.1 - Pregnancy - Teratogenicity studies have been performed in animals. Brimonidine tartrate was not teratogenic when given orally during gestation days 6 through 15 in rats and days 6 ...
-
10
OVERDOSAGE
There have been reports of inadvertent overdosage with timolol ophthalmic solution resulting in systemic effects similar to those seen with systemic beta-adrenergic blocking agents such as ...
-
11
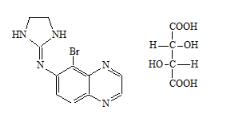
DESCRIPTION
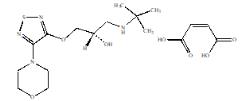
Brimonidine tartrate and timolol maleate ophthalmic solution 0.2%/0.5%, sterile, is a relatively selective alpha-2 adrenergic receptor agonist with a non-selective beta-adrenergic receptor ...
-
12
CLINICAL PHARMACOLOGY
12.1 - Mechanism of Action - Brimonidine tartrate and timolol maleate ophthalmic solution is comprised of two components: brimonidine tartrate and timolol. Each of these two components ...
-
13
NONCLINICAL TOXICOLOGY
13.1 - Carcinogenesis, Mutagenesis, Impairment of Fertility - With brimonidine tartrate, no compound-related carcinogenic effects were observed in either mice or rats following a ...
-
14
CLINICAL STUDIES
Clinical studies were conducted to compare the IOP-lowering effect over the course of the day of brimonidine tartrate and timolol maleate ophthalmic solution administered twice a day (BID) to ...
-
16
HOW SUPPLIED/STORAGE AND HANDLING
Brimonidine tartrate and timolol maleate ophthalmic solution is supplied sterile, in white opaque plastic LDPE bottles and tips, with blue high impact polystyrene (HIPS) caps as follows: 5 mL in ...
-
17
PATIENT COUNSELING INFORMATION
Patients with bronchial asthma, a history of bronchial asthma, severe chronic obstructive pulmonary disease, sinus bradycardia, second or third degree atrioventricular block, or cardiac failure ...
-
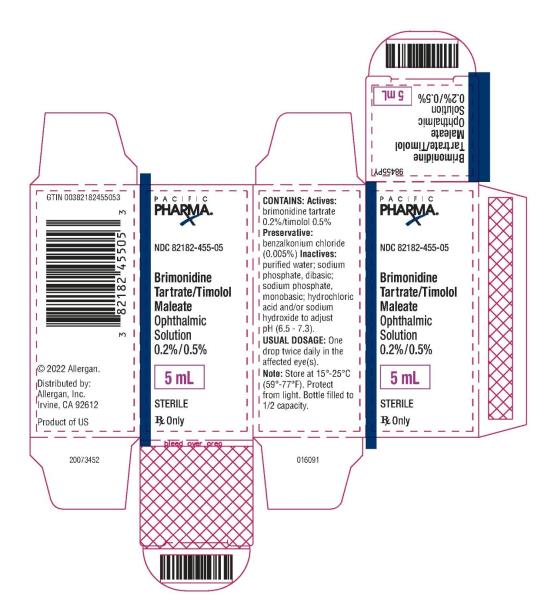
PRINCIPAL DISPLAY PANEL
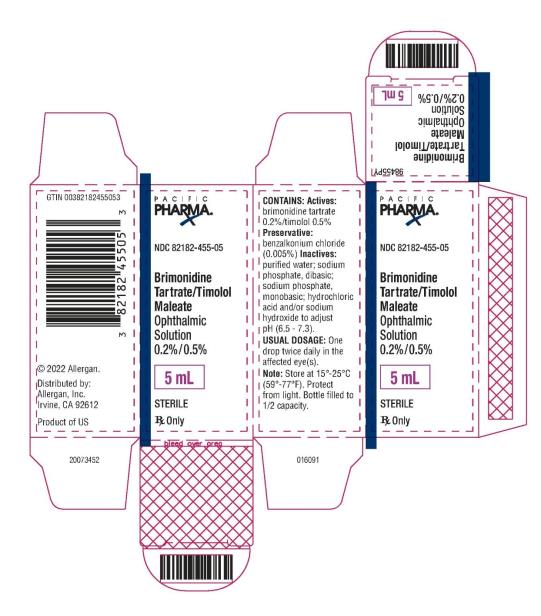
NDC 82182-455-05 - Brimonidine Tartrate/Timolol Maleate - Ophthalmic Solution 0.2%/0.5% 5 mL - STERILE - Rx Only - PACIFIC PHARMA
-
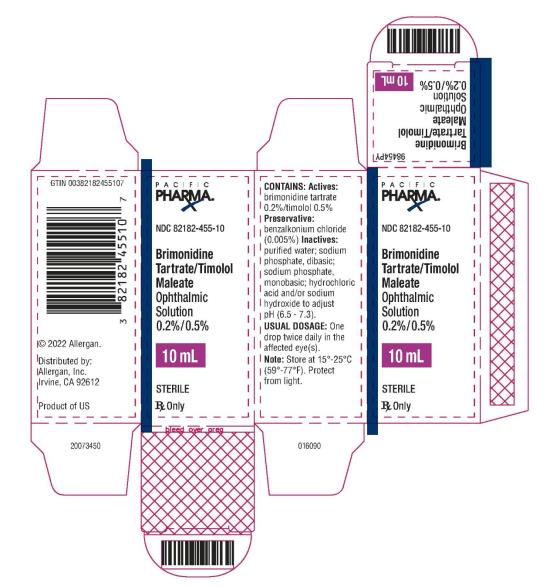
PRINCIPAL DISPLAY PANEL
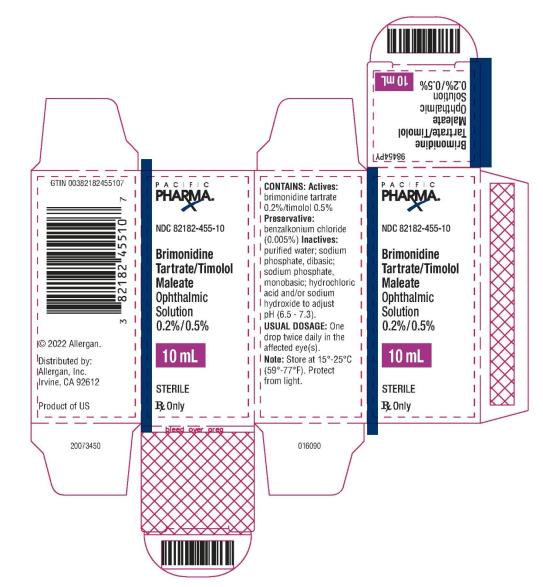
NDC 82182-455-10 - Brimonidine Tartrate/Timolol Maleate - Ophthalmic Solution 0.2%/0.5% 10 mL - STERILE - Rx Only - PACIFIC PHARMA
-
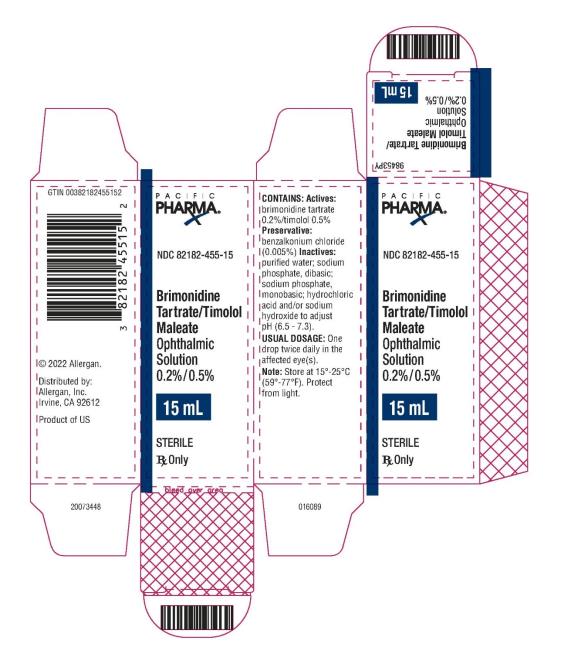
PRINCIPAL DISPLAY PANEL
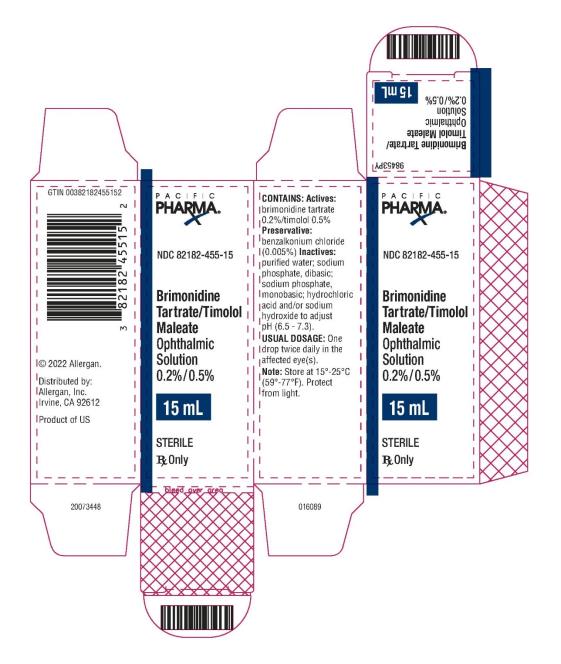
NDC 82182-455-10 - Brimonidine Tartrate/Timolol Maleate - Ophthalmic Solution 0.2%/0.5% 15 mL - STERILE - Rx Only - PACIFIC PHARMA
-
INGREDIENTS AND APPEARANCEProduct Information