Label: ZORYVE- roflumilast aerosol, foam
- NDC Code(s): 80610-430-60, 80610-430-93
- Packager: Arcutis Biotherapeutics, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated February 21, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZORYVE foam, 0.3%, safely and effectively. See full prescribing information for ZORYVE foam, 0.3%. ZORYVE® (roflumilast) topical ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEZORYVE® foam, 0.3%, is indicated for the treatment of seborrheic dermatitis in adult and pediatric patients 9 years of age and older.
-
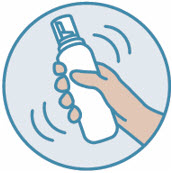
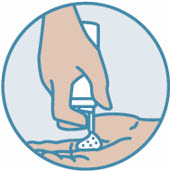
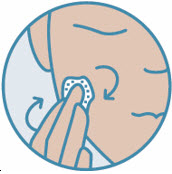
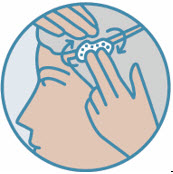
2 DOSAGE AND ADMINISTRATIONShake can prior to each use. Apply a thin layer of ZORYVE foam, 0.3%, once daily to affected areas on skin and/or scalp when they are not wet. Rub in completely. Wash hands after ...
-
3 DOSAGE FORMS AND STRENGTHSTopical foam, 0.3%: 3 mg of roflumilast per gram of white to off-white foam in 60-gram pressurized cans.
-
4 CONTRAINDICATIONSZORYVE foam, 0.3%, is contraindicated in patients with moderate to severe liver impairment (Child-Pugh B or C) [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
-
5 WARNINGS AND PRECAUTIONS5.1 Flammability - The propellants in ZORYVE foam, 0.3%, are flammable. Avoid fire, flame, and smoking during and immediately following application [see Dosage and Administration (2)].
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONSNo formal drug-drug interaction studies were conducted with ZORYVE foam, 0.3%. Drugs that Inhibit Cytochrome P450 (CYP) Enzymes - The co-administration of roflumilast with systemic CYP3A4 ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are insufficient data available on the use of ZORYVE foam, 0.3%, in pregnant women to inform a drug-associated risk of major birth defects, miscarriage, or ...
-
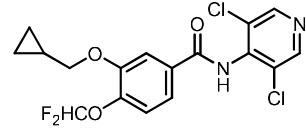
11 DESCRIPTIONZORYVE (roflumilast) topical foam, 0.3%, is a white to off-white foam for topical use. The active ingredient, roflumilast, is a phosphodiesterase 4 (PDE4) inhibitor. The chemical name of ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Roflumilast and its active metabolite (roflumilast N-oxide) are inhibitors of PDE4. Roflumilast and roflumilast N-oxide inhibition of PDE4 (a major cyclic ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Long-term studies were conducted in hamsters and mice with roflumilast to evaluate its carcinogenic potential. In 2-year oral gavage ...
-
14 CLINICAL STUDIESTwo randomized, double-blind, vehicle-controlled trials (STRATUM [NCT04973228] and Trial 203 [NCT04091646]) enrolled a total of 683 adult and pediatric subjects with seborrheic dermatitis ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGHow Supplied - ZORYVE (roflumilast) topical foam, 0.3%, is a white to off-white foam. It is supplied in a 60-gram pressurized aluminum can (NDC 80610-430-60). Storage and Handling - Store ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient or caregiver to read the FDA-approved patient labeling (Patient Information). Flammability - Because the propellants in ZORYVE foam, 0.3%, are flammable, instruct the patient ...
-
SPL UNCLASSIFIED SECTIONMarketed by: Arcutis Biotherapeutics, Inc. Westlake Village, CA 91361 - For more information, call 1-805-418-5006 or visit http://www.zoryve.com. © 2023 Arcutis Biotherapeutics, Inc. All rights ...
-
PATIENT PACKAGE INSERTPatient Information - ZORYVE® (zor-EEV) (roflumilast) topical foam, 0.3% This Patient Information has been approved by the U.S. Food and Drug Administration.Issued: 12/2023 - Important ...
-
INSTRUCTIONS FOR USE ZORYVE® (zor-EEV) (roflumilast) topical foam, 0.3%This Instructions for Use contains information on how to apply ZORYVE foam. Read this Instructions for Use before you start using ZORYVE foam and each time you get a refill. There may be new ...
-
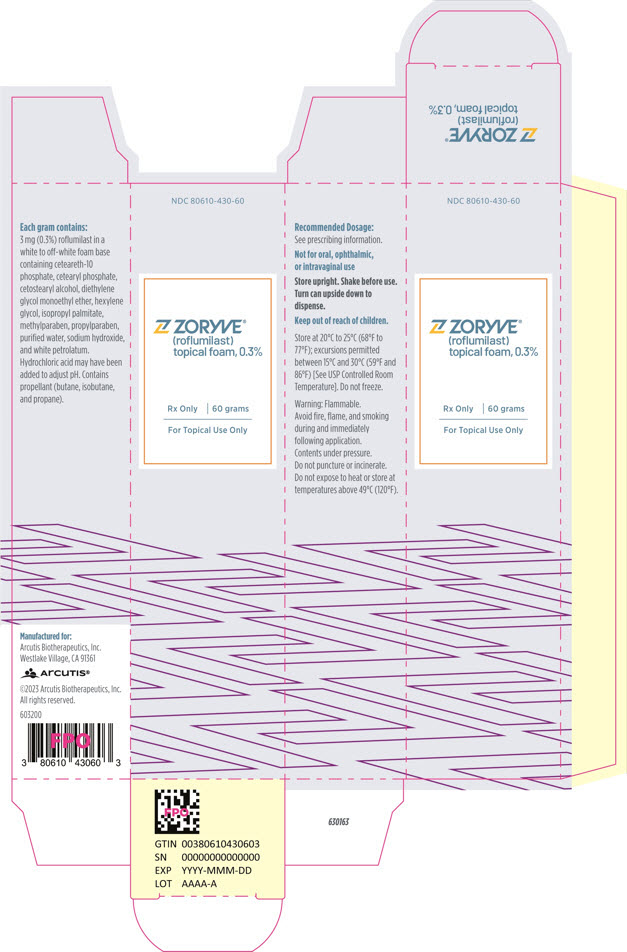
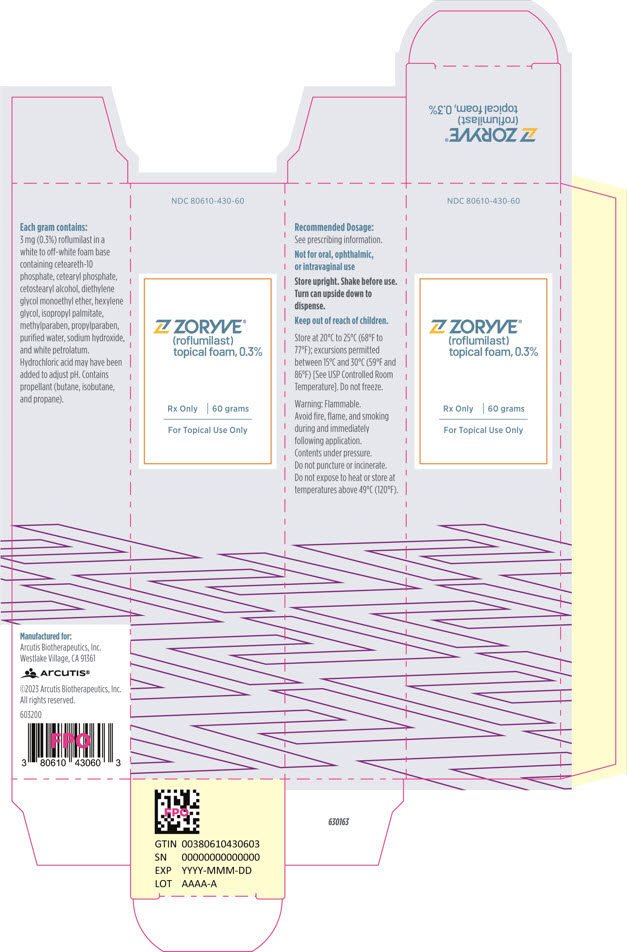
PRINCIPAL DISPLAY PANEL - 60 g Can CartonNDC 80610-430-60 - ZORYVE® (roflumilast) topical foam, 0.3% Rx Only - 60 grams - For Topical Use Only
-
INGREDIENTS AND APPEARANCEProduct Information

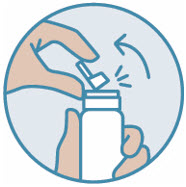 Gently pull back on the nozzle to break the plastic piece at the base.
Gently pull back on the nozzle to break the plastic piece at the base.