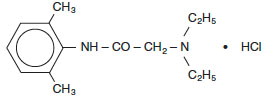Label: MLK KIT F2- marcaine, lidocaine, kenalog, povidone iodine kit
- NDC Code(s): 0409-1162-18, 55150-165-05, 67777-419-02, 70121-1049-5, view more
- Packager: Asclemed USA, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use BUPIVACAINE HYDROCHLORIDE INJECTION safely and effectively. See full prescribing information for BUPIVACAINE HYDROCHLORIDE ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)
WARNING: RISK OF CARDIAC ARREST WITH USE OF BUPIVACAINE HYDROCHLORIDE INJECTION IN OBSTETRICAL ANESTHESIA
There have been reports of cardiac arrest with difficult resuscitation or death during use of Bupivacaine Hydrochloride Injection for epidural anesthesia in obstetrical patients. In most cases, this has followed use of the 0.75% (7.5 mg/mL) concentration. Resuscitation has been difficult or impossible despite apparently adequate preparation and appropriate management. Cardiac arrest has occurred after convulsions resulting from systemic toxicity, presumably following unintentional intravascular injection. The 0.75% (7.5 mg/mL) concentration of Bupivacaine Hydrochloride Injection is not recommended for obstetrical anesthesia and should be reserved for surgical procedures where a high degree of muscle relaxation and prolonged effect are necessary [see Warnings and Precautions (5.1)].
Close -
1 INDICATIONS AND USAGEBupivacaine Hydrochloride Injection is indicated in adults for the production of local or regional anesthesia or analgesia for surgery, dental and oral surgery procedures, diagnostic and ...
-
2 DOSAGE AND ADMINISTRATION2.1 Important Dosage and Administration Information - Bupivacaine Hydrochloride Injection is not for intrathecal use. Discard unused portions of solution not containing preservatives, i.e. ...
-
3 DOSAGE FORMS AND STRENGTHSBupivacaine Hydrochloride Injection, USP is a clear, colorless solution available as: 0.5% (50 mg/10 mL) (5 mg/mL) in single-dose teartop vials.
-
4 CONTRAINDICATIONSBupivacaine Hydrochloride Injection is contraindicated in: obstetrical paracervical block anesthesia. Its use in this technique has resulted in fetal bradycardia and death. intravenous regional ...
-
5 WARNINGS AND PRECAUTIONS5.1 Risk of Cardiac Arrest with Use of Bupivacaine Hydrochloride Injection in Obstetrical Anesthesia - There have been reports of cardiac arrest with difficult resuscitation or death during use ...
-
6 ADVERSE REACTIONSThe following clinically significant adverse reactions have been reported and described in the Warnings and Precautions section of the labeling: Cardiac Arrest in Obstetrical Anesthesia ...
-
7 DRUG INTERACTIONS7.1 Local Anesthetics - The toxic effects of local anesthetics are additive. If coadministration of other local anesthetics with Bupivacaine Hydrochloride Injection cannot be avoided, monitor ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Bupivacaine Hydrochloride Injection is contraindicated for obstetrical paracervical block anesthesia. Its use in this technique has resulted in fetal bradycardia ...
-
10 OVERDOSAGEClinical Presentation - Acute emergencies from use of Bupivacaine Hydrochloride Injection are generally related to high plasma levels encountered during therapeutic use or to unintended ...
-
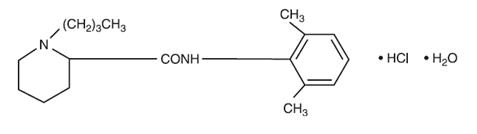
11 DESCRIPTIONBupivacaine Hydrochloride Injection contains bupivacaine hydrochloride, an amide local anesthetic, as the active pharmaceutical ingredient. The route of administration for Bupivacaine ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Bupivacaine blocks the generation and the conduction of nerve impulses, presumably by increasing the threshold for electrical excitation in the nerve, by slowing the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Long-term studies in animals to evaluate the carcinogenic potential of bupivacaine hydrochloride have not been ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGStore at 20 °C to 25 °C (68 °F to 77 °F); excursions permitted between 15 °C to 30 °C (59 °F to 86 °F). [See USP Controlled Room Temperature.] Bupivacaine Hydrochloride Injection, USP ...
-
17 PATIENT COUNSELING INFORMATIONAllergic-Type Reactions - Assess if the patient has had allergic-type reactions to amide-type local anesthetics - [see - Contraindications (4), Adverse Reactions (6)] ...
-
SPL UNCLASSIFIED SECTIONThis product's labeling may have been updated. For the most recent prescribing information, please visit - www.pfizer.com. Distributed by Hospira, Inc., Lake Forest, IL 60045 ...
-
SPL UNCLASSIFIED SECTIONLIDOCAINE HYDROCHLORIDE INJECTION, USP - For Infiltration and Nerve Block Including Caudal and Epidural Use. Preservative-Free - Rx only
-
DESCRIPTIONLidocaine hydrochloride injection, USP is sterile, nonpyrogenic, aqueous solution that contains a local anesthetic agent and is administered parenterally by injection. See - INDICATIONS AND ...
-
CLINICAL PHARMACOLOGYMechanism of Action - Lidocaine hydrochloride stabilizes the neuronal membrane by inhibiting the ionic fluxes required for the initiation and conduction of impulses thereby effecting local ...
-
INDICATIONS AND USAGELidocaine hydrochloride injection is indicated for production of local or regional anesthesia by infiltration techniques such as percutaneous injection and intravenous regional anesthesia by ...
-
CONTRAINDICATIONSLidocaine hydrochloride is contraindicated in patients with a known history of hypersensitivity to local anesthetics of the amide type.
-
WARNINGSLIDOCAINE HYDROCHLORIDE INJECTION FOR INFILTRATION AND NERVE BLOCK SHOULD BE EMPLOYED ONLY BY CLINICIANS WHO ARE WELL VERSED IN DIAGNOSIS AND MANAGEMENT OF DOSE-RELATED TOXICITY AND OTHER ACUTE ...
-
PRECAUTIONSGeneral - The safety and effectiveness of lidocaine hydrochloride depend on proper dosage, correct technique, adequate precautions, and readiness for emergencies. Standard textbooks should be ...
-
ADVERSE REACTIONSSystemic - Adverse experiences following the administration of lidocaine hydrochloride are similar in nature to those observed with other amide local anesthetic agents. These adverse ...
-
OVERDOSAGEAcute emergencies from local anesthetics are generally related to high plasma levels encountered during therapeutic use of local anesthetics or to unintended subarachnoid injection of local ...
-
DOSAGE AND ADMINISTRATIONTable 1 (Recommended Dosages) summarizes the recommended volumes and concentrations of lidocaine hydrochloride injection for various types of anesthetic procedures. The dosages suggested in this ...
-
MAXIMUM RECOMMENDED DOSAGESAdults - For normal healthy adults, the maximum individual dose should not exceed 4.5 mg/kg (2 mg/lb) of body weight, and in general it is recom mended that the max i m um t otal dose does ...
-
STERILIZATION, STORAGE AND TECHNICAL PROCEDURESDisinfecting agents containing heavy metals, which cause release of respective ions (mercury, zinc, copper, etc.) should not be used for skin or mucous membrane disinfection as they have been ...
-
HOW SUPPLIEDLidocaine Hydrochloride Injection, USP is supplied as follows: Lidocaine Hydrochloride Injection USP, 2% (20 mg/mL) 5 mL Single-Dose Vials in a Carton of ...
-
SPL UNCLASSIFIED SECTIONTriamcinolone Acetonide Injectable Suspension, USP - NOT FOR USE IN NEONATES - CONTAINS BENZYL ALCOHOL - For Intramuscular or Intra-articular Use Only - NOT FOR INTRAVENOUS, INTRADERMAL ...
-
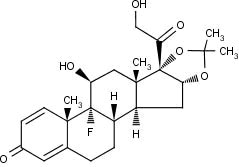
DESCRIPTIONTriamcinolone acetonide injectable suspension, USP is a synthetic glucocorticoid corticosteroid with anti-inflammatory action. THIS FORMULATION IS SUITABLE FOR INTRAMUSCULAR AND INTRA-ARTICULAR ...
-
CLINICAL PHARMACOLOGYGlucocorticoids, naturally occurring and synthetic, are adrenocortical steroids that are readily absorbed from the gastrointestinal tract. Naturally occurring glucocorticoids (hydrocortisone and ...
-
INDICATIONS AND USAGEIntramuscular - Where oral therapy is not feasible, injectable corticosteroid therapy, including triamcinolone acetonide injectable suspension is indicated - for intramuscularuseas follows ...
-
CONTRAINDICATIONSTriamcinolone acetonide injectable suspension is contraindicated in patients who are hypersensitive to any components of this product (see - WARNINGS: General). Intramuscular corticosteroid ...
-
WARNINGSSerious Neurologic Adverse Reactions with Epidural Administration - Serious neurologic events, some resulting in death, have been reported with epidural injection of corticosteroids (see ...
-
PRECAUTIONSGeneral - This product, like many other steroid formulations, is sensitive to heat. Therefore, it should not be autoclaved when it is desirable to sterilize the exterior of the vial. The lowest ...
-
ADVERSE REACTIONS(listed alphabetically under each subsection) The following adverse reactions may be associated with corticosteroid therapy: Allergic reactions:Anaphylaxis including death, angioedema ...
-
OVERDOSAGETreatment of acute overdosage is by supportive and symptomatic therapy. For chronic overdosage in the face of severe disease requiring continuous steroid therapy, the dosage of the corticosteroid ...
-
DOSAGE AND ADMINISTRATIONGeneral - NOTE: CONTAINS BENZYL ALCOHOL (see - PRECAUTIONS). The initial dose of triamcinolone acetonide injectable suspension may vary from 2.5 mg to 100 mg per day depending on the ...
-
HOW SUPPLIEDTriamcinolone Acetonide Injectable Suspension, USP is supplied in vials providing 40 mg triamcinolone acetonide per mL. 40 mg/mL, 1 mL - 1 mL single-dose vial: NDC 70121-1049-1 - 25 single-dose ...
- Povidone Iodine Swabsticks
-
ACTIVE INGREDIENTActive Ingredient Purpose - Povidone Iodine 10% w/v (9.85% w/w/) Antiseptic
-
Purpose:Purpose: First aid antiseptic to help prevent skin infection in minor cuts, scrapes and burns. For preparation of the skin prior to surgery. Helps reduce bacteria that can potentially ...
-
Warnings:FOR EXTERNAL USE ONLY
-
Do not use:As a first aid antiseptic for more than 1 week. In the eyes. Over large areas of the body.
-
Ask a doctor before use if you have:Deep puncture wounds - Animal bites - Serious burns
-
Stop Use:If irritation and redness develop - If condition persists for more than 72 hours, consult a physician.
-
Keep Out Of Reach Of ChildrenKeep out of reach of children.If swallowed, get medical help or contact a Poison Control Center.
-
Directions Povidone iodine:Tear at notch, remove applicator, use only once. As a first aid antiseptic - clean affected area - apply 1 to 3 times daily - may be covered with a sterile bandage, if bandaged let dry. For ...
-
Other information:Store at room temperature. Avoid excessive heat
-
INDICATIONS & USAGEFor use as an - first aid antiseptic - pre-operative skin preperation
-
Inactive IngredientsInactive ingredients: Citric acid, glycerin, polysorbate 80, sodium citrate USP, sodium phosphate dibasic, water
-
PRINCIPAL DISPLAY PANELPRINCIPAL DISPLAY PANEL - NDC: 76420-742-01 - Rx Only - MLK F2 Kit™ Kit Contains - 1 Bupivacaine HCI 0.5% Single Dose Vial (10mL) 1 Lidocaine HCI Injection, USP 2% (5mL) 3 Triamcinolone Acetonide ...
-
INGREDIENTS AND APPEARANCEProduct Information