Label: SOTALOL HYDROCHLORIDE AF- sotalol hydrochloride tables af tablet
SOTALOL HYDROCHLORIDE AF- sotalol hydrochloride tablets ...view full title
SOTALOL HYDROCHLORIDE AF- sotalol hydrochloride tablets ...
- NDC Code(s): 76385-125-01, 76385-125-50, 76385-126-01, 76385-126-50, view more
- Packager: Bayshore Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
To minimize the risk of induced arrhythmia, patients initiated or re-initiated on Sotalol Hydrochloride Tablets (AF) should be placed for a minimum of three days (on their maintenance dose) in a facility that can provide cardiac resuscitation, continuous electrocardiographic monitoring and calculations of creatinine clearance. For detailed instructions regarding dose selection, and special cautions for people with renal impairment, see DOSAGE AND ADMINISTRATION. Sotalol is also indicated for the treatment of documented life threatening ventricular arrhythmias and is marketed under the brand name Betapace® (sotalol hydrochloride tablets). Sotalol hydrochloride tablets, however, should not be substituted for sotalol hydrochloride tablets (AF) because of significant differences in labeling (i.e., patient package insert, dosing administration and safety information).
Close -
SPL UNCLASSIFIED SECTIONRx only
-
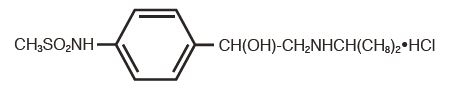
DESCRIPTIONSotalol Hydrochloride Tablets, USP (AF) is an antiarrhythmic drug with Class II (beta-adrenoreceptor blocking) and Class III (cardiac action potential duration prolongation) properties. It is ...
-
CLINICAL PHARMACOLOGYMechanism of Action - Sotalol AF has both beta-adrenoreceptor blocking (Vaughan Williams Class II) and cardiac action potential duration prolongation (Vaughan Williams Class III) antiarrhythmic ...
-
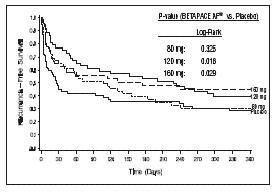
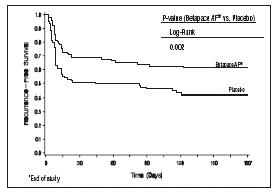
Clinical StudiesProlongation of Time to Recurrence of Symptomatic Atrial Fibrillation/ Flutter - Sotalol AF has been studied in patients with symptomatic AFIB/AFL in two principal studies, one in patients with ...
-
INDICATIONS AND USAGESotalol AF are indicated for the maintenance of normal sinus rhythm [delay in time to recurrence of atrial fibrillation/atrial flutter (AFIB/AFL)] in patients with symptomatic AFIB/AFL who are ...
-
CONTRAINDICATIONSSotalol AF is contraindicated in patients with sinus bradycardia (<50 bpm during waking hours), sick sinus syndrome or second and third degree AV block (unless a functioning pacemaker is present) ...
-
WARNINGSVentricular Arrhythmia - Sotalol AF can cause serious ventricular arrhythmias, primarily Torsade de Pointes (TdP) type ventricular tachycardia, a polymorphic ventricular tachycardia associated ...
-
PRECAUTIONSRenal Impairment - Sotalol AF is eliminated principally via the kidneys through glomerular filtration and to a small degree by tubular secretion. There is a direct relationship between renal ...
-
Information for PatientsPlease refer to the patient package insert. Prior to initiation of Sotalol AF therapy, the patient should be advised to read the patient package insert and reread it each time therapy is ...
-
Drug InteractionsDrugs undergoing CYP450 metabolism - Sotalol is primarily eliminated by renal excretion; therefore, drugs that are metabolized by CYP450 are not expected to alter the pharmacokinetics of sotalol ...
-
Drug/Laboratory Test InteractionsThe presence of sotalol in the urine may result in falsely elevated levels of urinary metanephrine when measured by fluorimetric or photometric methods. In screening patients suspected of having a ...
-
Carcinogenesis, Mutagenesis, Impairment of FertilityNo evidence of carcinogenic potential was observed in rats during a 24-month study at 137 to 275 mg/kg/ day (approximately 30 times the maximum recommended human oral dose (MRHD) as mg/kg or 5 ...
-
Pregnancy Category BReproduction studies in rats and rabbits during organogenesis at 100 and 22 times the MRHD as mg/kg (9 and 7 times the MRHD as mg/m2), respectively, did not reveal any teratogenic potential ...
-
Nursing MothersSotalol is excreted in the milk of laboratory animals and has been reported to be present in human milk. Because of the potential for adverse reactions in nursing infants from Sotalol AF, a ...
-
Pediatric UseThe safety and effectiveness of Sotalol AF in children have not been established. However, the Class III electrophysiologic and beta-blocking effects, the pharmacokinetics, and the relationship ...
-
ADVERSE REACTIONSAdverse events that are clearly related to Sotalol AF are those which are typical of its Class II (beta-blocking) and Class III (cardiac action potential duration prolongation) effects. The common ...
-
OVERDOSAGEIntentional or accidental overdosage with sotalol has rarely resulted in death. Symptoms and Treatment of Overdosage - The most common signs to be expected are bradycardia, congestive heart ...
-
DOSAGE AND ADMINISTRATIONDosing and Administration in Adults - Therapy with Sotalol AF must be initiated (and, if necessary, titrated) in a setting that provides continuous electrocardiographic (ECG) monitoring and in ...
-
HOW SUPPLIEDSotalol Hydrochloride Tablets, USP (AF) are presented as follows: For 80 mg strength: White to off-white colored, capsule shaped, scored tablets debossed 'B107' on one side and scored on the ...
-
PATIENT INFORMATIONWhat You Should Know About Sotalol AF - (generic name: sotalol hydrochloride) This summary contains important patient information that has been reviewed and approved by the U.S. Food and Drug ...
-
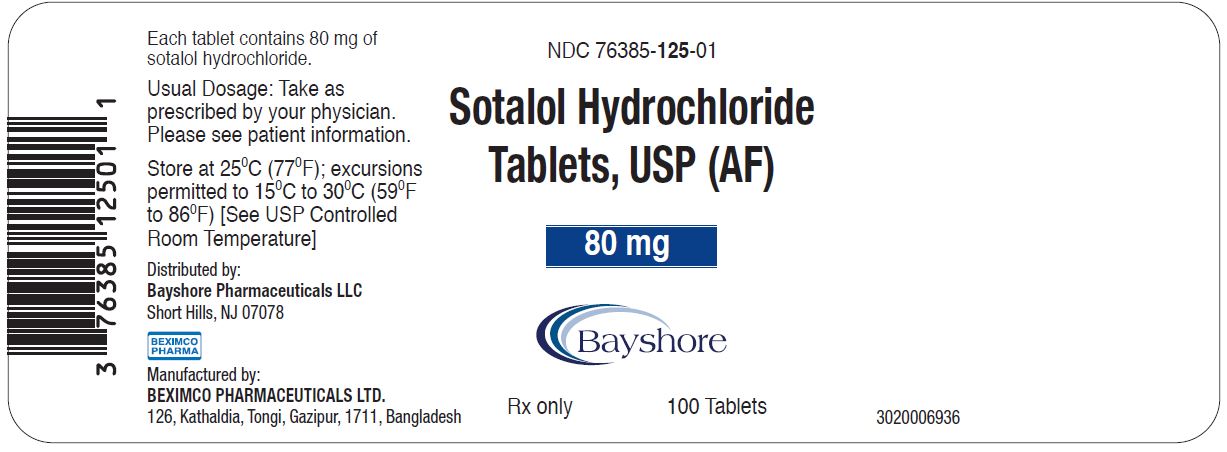
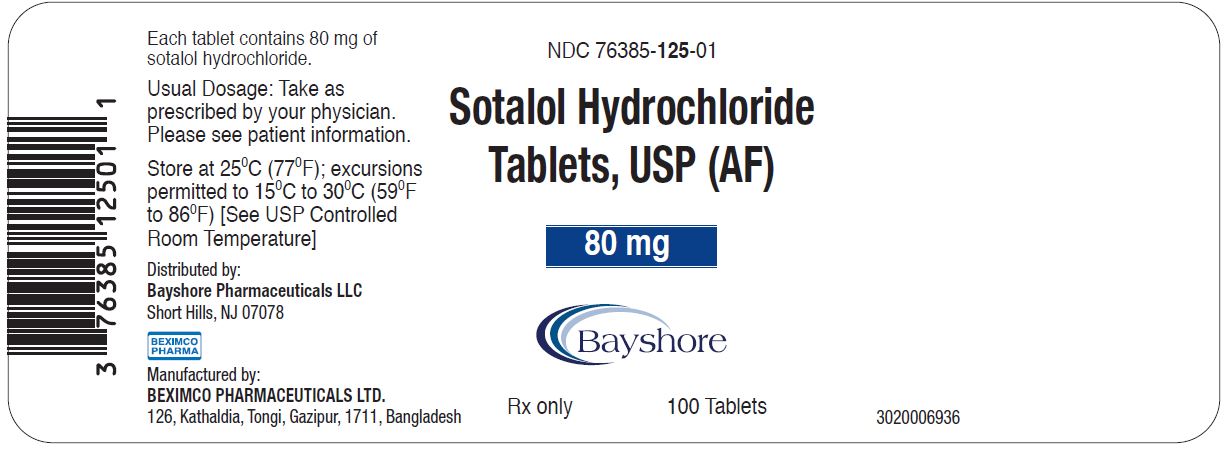
PRINCIPAL DISPLAY PANEL - 80 mg container labelNDC 76385-125-01 - 100 Tablets - Sotalol Hydrochloride Tablets, USP (AF) 80 mg Rx only - Distributed by: Bayshore Pharmaceuticals LLC - Short Hills, NJ 07078 - Manufactured by - BEXIMCO ...
-
PRINCIPAL DISPLAY PANEL - 120 mg container labelNDC 76385-126-01 - 100 Tablets - Sotalol Hydrochloride Tablets, USP (AF) 120 mg Rx only - Distributed by: Bayshore Pharmaceuticals LLC - Short Hills, NJ 07078 - Manufactured by - BEXIMCO ...
-
PRINCIPAL DISPLAY PANEL - 160 mg container labelNDC 76385-127-01 - 100 Tablets - Sotalol Hydrochloride Tablets, USP (AF) 160 mg Rx only - Distributed by: Bayshore Pharmaceuticals LLC - Short Hills, NJ 07078 - Manufactured by - BEXIMCO ...
-
INGREDIENTS AND APPEARANCEProduct Information
View Labeling Archives for this drug
SOTALOL HYDROCHLORIDE AF- sotalol hydrochloride tables af tablet
SOTALOL HYDROCHLORIDE AF- sotalol hydrochloride tablets ...view full title
SOTALOL HYDROCHLORIDE AF- sotalol hydrochloride tablets ...
Number of versions: 2
RxNorm
SOTALOL HYDROCHLORIDE AF- sotalol hydrochloride tables af tablet
SOTALOL HYDROCHLORIDE AF- sotalol hydrochloride tablets ...view full title
SOTALOL HYDROCHLORIDE AF- sotalol hydrochloride tablets ...
Get Label RSS Feed for this Drug
SOTALOL HYDROCHLORIDE AF- sotalol hydrochloride tables af tablet
SOTALOL HYDROCHLORIDE AF- sotalol hydrochloride tablets ...view full title
SOTALOL HYDROCHLORIDE AF- sotalol hydrochloride tablets ...
NDC Codes
SOTALOL HYDROCHLORIDE AF- sotalol hydrochloride tables af tablet
SOTALOL HYDROCHLORIDE AF- sotalol hydrochloride tablets ...view full title
SOTALOL HYDROCHLORIDE AF- sotalol hydrochloride tablets ...