Label: ETODOLAC tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 76385-118-01, 76385-119-01 - Packager: Bayshore Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONETODOLAC TABLETS USP - Bayshore Pharmaceuticals LLC. Rx only
-
BOXED WARNING
(What is this?)Cardiovascular Thrombotic Events - Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke ...
Cardiovascular Thrombotic Events
- Nonsteroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use [See WARNINGS and PRECAUTIONS].
- Etodolac tablets, 400 mg and 500 mg are contraindicated in the setting of coronary artery bypass graft (CABG) surgery [See CONTRAINDICATIONS and WARNINGS.]
CloseGastrointestinal Risk
- NSAIDs cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal (GI) events. (See WARNINGS.)
-
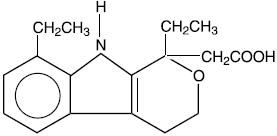
DESCRIPTIONEtodolac tablets, USP are members of the pyranocarboxylic acid group of nonsteroidal anti-inflammatory drugs (NSAIDs). Each tablet contains etodolac for oral administration. Etodolac is a racemic ...
-
CLINICAL PHARMACOLOGYPharmacodynamics - Etodolac is a nonsteroidal anti-inflammatory drug (NSAID) that exhibits anti-inflammatory, analgesic, and antipyretic activities in animal models. The mechanism of action of ...
-
CLINICAL TRIALSAnalgesia - Controlled clinical trials in analgesia were single-dose, randomized, double-blind, parallel studies in three pain models, including dental extractions. The analgesic effective dose ...
-
INDICATIONS AND USAGECarefully consider the potential benefits and risks of etodolac tablets and other treatment options before deciding to use etodolac tablets. Use the lowest effective dose for the shortest duration ...
-
CONTRAINDICATIONSEtodolac tablets are contraindicated in patients with known hypersensitivity to etodolac or other ingredients in etodolac tablets. Etodolac tablets should not be given to patients who have ...
-
WARNINGSCARDIOVASCULAR EFFECTS - Cardiovascular Thrombotic Events - Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of ...
-
PRECAUTIONSGeneral - Etodolac tablets cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease ...
-
ADVERSE REACTIONSIn patients taking etodolac tablets or other NSAIDs, the most frequently reported adverse experiences occurring in approximately 1-10% of patients are: Gastrointestinal experiences including ...
-
OVERDOSAGESymptoms following acute NSAID overdose are usually limited to lethargy, drowsiness, nausea, vomiting, and epigastric pain, which are generally reversible with supportive care. Gastrointestinal ...
-
DOSAGE AND ADMINISTRATIONCarefully consider the potential benefits and risks of etodolac tablets and other treatment options before deciding to use etodolac tablets. Use the lowest effective dose for the shortest duration ...
-
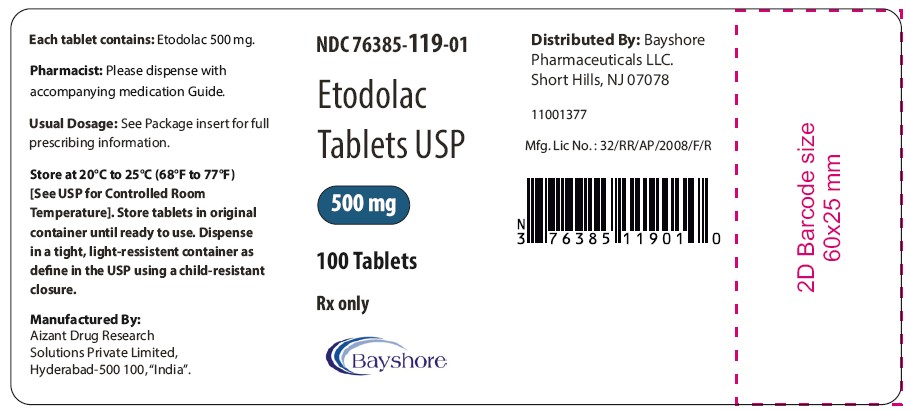
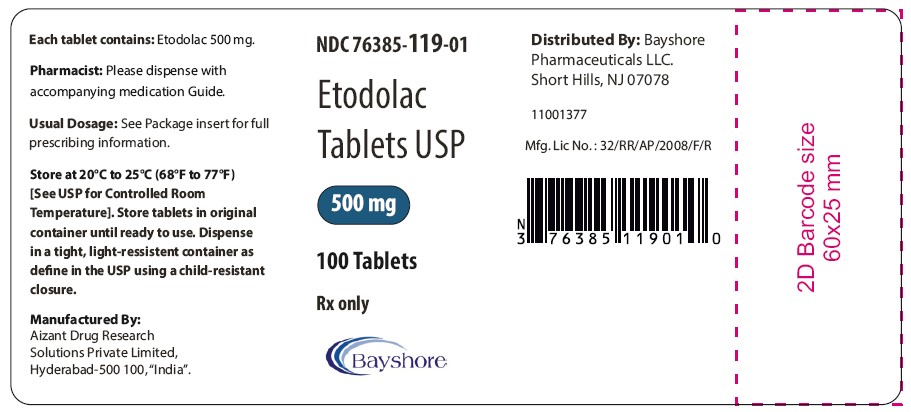
HOW SUPPLIEDEtodolac tablets USP, 400 mg are available as beige colored, oval shaped tablets debossed BY7 on one side and plain on other side. - in bottles of 100 NDC 76385-118-01 - Etodolac tablets USP, 500 ...
-
Medication Guide for Nonsteroidal Anti-inflammatory Drugs (NSAIDs)What is the most important information I should know about medicines called Nonsteroidal Anti-inflammatory Drugs (NSAIDs)? NSAIDs can cause serious side effects, including: Increased risk of ...
-
PRINCIPAL DISPLAY PANEL - 400 mg Container LabelNDC 76385-118-01 - Etodolac - Tablets USP - 400 mg - 100 Tablets - Rx only
-
PRINCIPAL DISPLAY PANEL - 500 mg Container LabelNDC 76385-119-01 - Etodolac - Tablets USP - 500 mg - 100 Tablets - Rx only
-
INGREDIENTS AND APPEARANCEProduct Information