Label: BENZTROPINE MESYLATE tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 76385-103-01, 76385-103-10, 76385-104-01, 76385-104-10, view more - Packager: Bayshore Pharmaceuticals LLC (NJ)
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 26, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
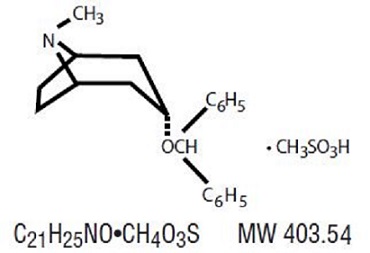
DESCRIPTIONBenztropine Mesylate is a synthetic compound containing structural features found in atropine and diphenhydramine. It is a crystalline white powder, very soluble in water, designated as ...
-
CLINICAL PHARMACOLOGYBenztropine mesylate possesses both anticholinergic and antihistaminic effects, although only the former have been established as therapeutically significant in the management of parkinsonism. In ...
-
INDICATIONS AND USAGEFor use as an adjunct in the therapy of all forms of parkinsonism. Useful also in the control of extrapyramidal disorders (except tardive dyskinesia - see PRECAUTIONS) due to neuroleptic drugs ...
-
CONTRAINDICATIONSHypersensitivity to benztropine mesylate tablets. Because of its atropine-like side effects, this drug is contraindicated in pediatric patients under three years of age, and should be used with ...
-
WARNINGSSafe use in pregnancy has not been established. Benztropine mesylate may impair mental and/or physical abilities required for performance of hazardous tasks, such as operating machinery or ...
-
PRECAUTIONSGeneralSince benztropine mesylate has cumulative action, continued supervision is advisable. Patients with a tendency to tachycardia and patients with prostatic hypertrophy should be observed closely ...
-
Drug Interactions
Antipsychotic drugs such as phenothiazines or haloperidol; tricyclic antidepressants (see WARNINGS).
-
Pediatric Use
Because of the atropine-like side effects, benztropine mesylate should be used with caution in pediatric patients over three years of age (see CONTRAINDICATIONS).
-
ADVERSE REACTIONSThe adverse reactions below, most of which are antichlolinergic in nature, have been reported and within each category are listed in order of decreasing severity. Cardiovascular ...
-
OVERDOSAGEManifestations - May be any of those seen in atropine poisoning or antihistamine overdosage: CNS depression, preceded or followed by stimulation; confusion; nervousness; listlessness ...
-
DOSAGE AND ADMINISTRATIONBenztropine mesylate tablets should be used when patients are able to take oral medication. Because of cumulative action, therapy should be initiated with a low dose which is increased gradually ...
-
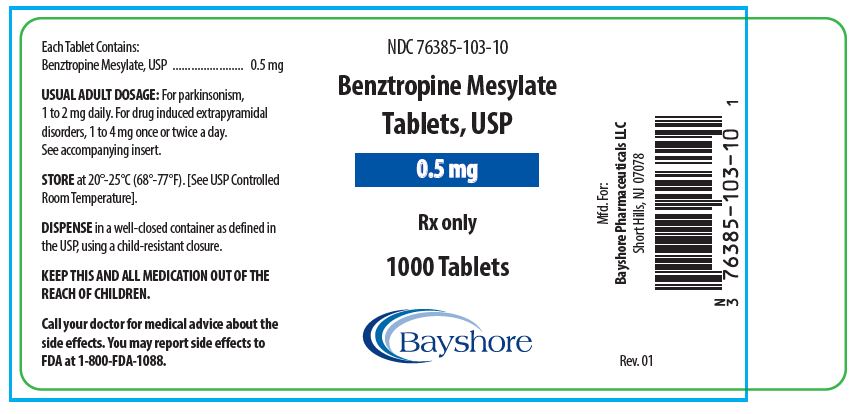
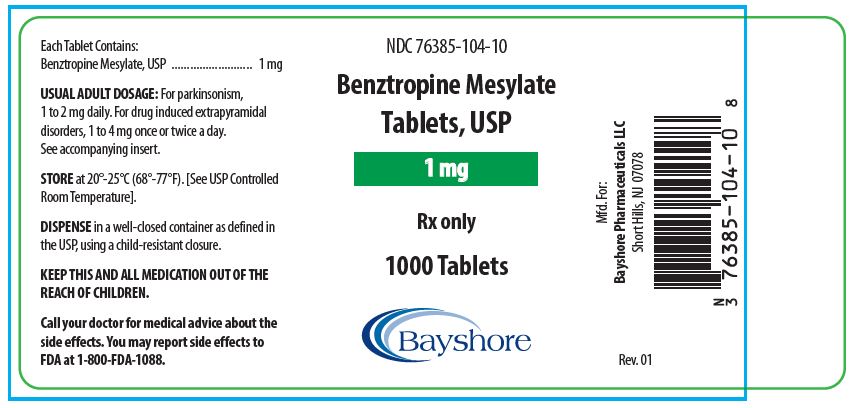
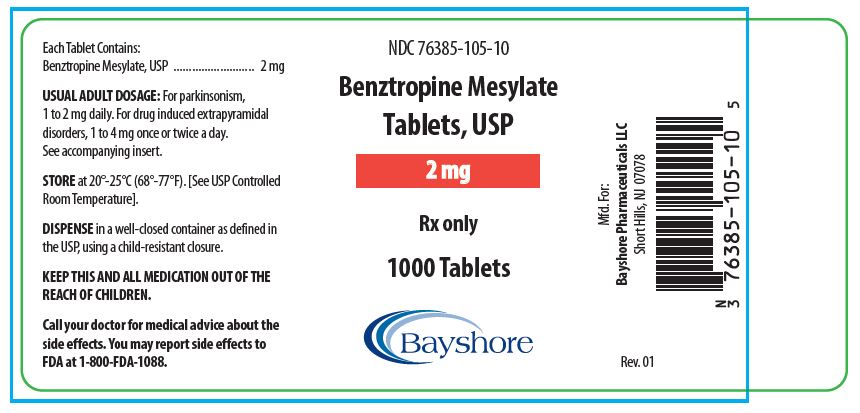
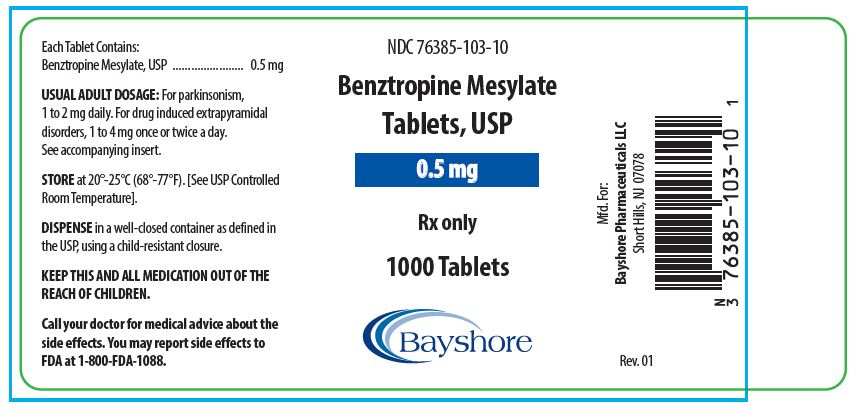
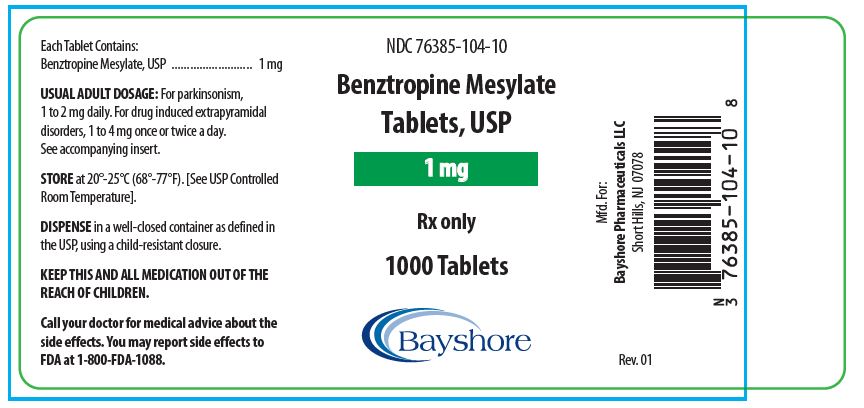
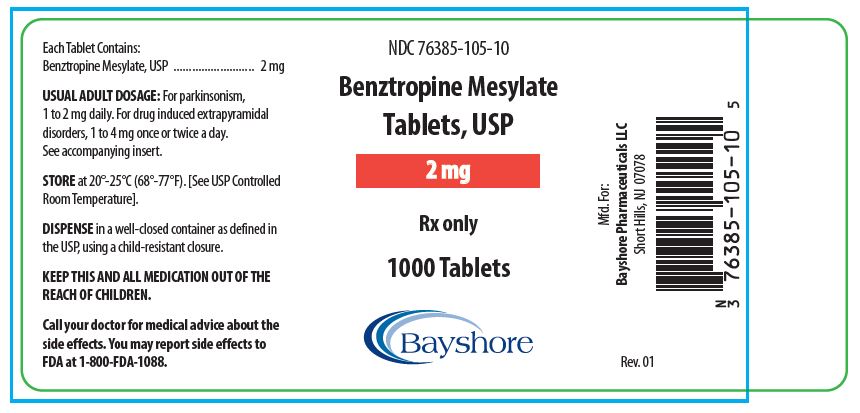
HOW SUPPLIEDBenztropine Mesylate Tablets, USP are available as follows: 0.5 mg white, round, bisected, compressed tablets, debossed “EP 136”, in bottles of 100 (NDC 76385-103-01) and 1000 (NDC 76385-103-10 ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
 ...
... -
INGREDIENTS AND APPEARANCEProduct Information