Label: CINACALCET tablet
- NDC Code(s): 76282-674-30, 76282-675-30, 76282-676-30
- Packager: EXELAN PHARMACEUTICALS INC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 14, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CINACALCET HYDROCHLORIDE TABLETS safely and effectively. See full prescribing information for CINACALCET HYDROCHLORIDE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Secondary Hyperparathyroidism - Cinacalcet hydrochloride tablets are indicated for the treatment of secondary hyperparathyroidism (HPT) in adult patients with chronic kidney disease (CKD) on ...
-
2 DOSAGE AND ADMINISTRATION2.1 Administration - Cinacalcet hydrochloride tablets should be taken with food or shortly after a meal. Cinacalcet hydrochloride tablets are administered orally and should always be taken ...
-
3 DOSAGE FORMS AND STRENGTHSCinacalcet Hydrochloride Tablets 30 mg: Light green colored, oval shaped, biconvex film coated tablets debossed with 'CL' on one side and '410' on other side. Cinacalcet Hydrochloride Tablets ...
-
4 CONTRAINDICATIONSCinacalcet hydrochloride tablets treatment initiation is contraindicated if serum calcium is less than the lower limit of the normal range [see Warnings and Precautions (5.1)].
-
5 WARNINGS AND PRECAUTIONS5.1 Hypocalcemia - Cinacalcet hydrochloride tablets lowers serum calcium and can lead to hypocalcemia [see Adverse Reactions (6.1)]. Significant lowering of serum calcium can cause paresthesias ...
-
6 ADVERSE REACTIONSThe following adverse reactions are discussed in greater detail in other sections of labeling: Hypocalcemia [see Warnings and Precautions (5.1)] Upper Gastrointestinal Bleeding [see ...
-
7 DRUG INTERACTIONS7.1 Strong CYP3A4 Inhibitors - Cinacalcet is partially metabolized by CYP3A4. Dose adjustment of cinacalcet hydrochloride tablets may be required if a patient initiates or discontinues therapy ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Limited case reports of cinacalcet hydrochloride use in pregnant women are insufficient to inform a drug associated risk of adverse developmental outcomes. In ...
-
10 OVERDOSAGEOverdosage of cinacalcet hydrochloride may lead to hypocalcemia. In the event of overdosage, patients should be monitored for signs and symptoms of hypocalcemia and appropriate measures taken to ...
-
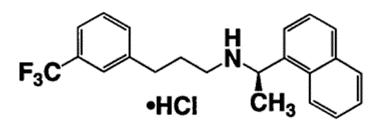
11 DESCRIPTIONCinacalcet hydrochloride tablets contain the hydrochloride salt of cinacalcet, a positive modulator of the calcium sensing receptor. The empirical formula for cinacalcet is C22H22F3N⋅HCl with a ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The calcium-sensing receptor on the surface of the chief cell of the parathyroid gland is the principal regulator of PTH synthesis and secretion. Cinacalcet, the ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity - Standard lifetime dietary carcinogenicity bioassays were conducted in mice and rats. Mice were given cinacalcet at ...
-
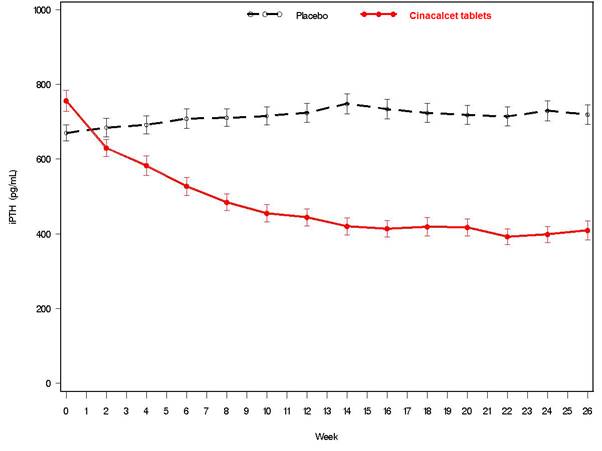
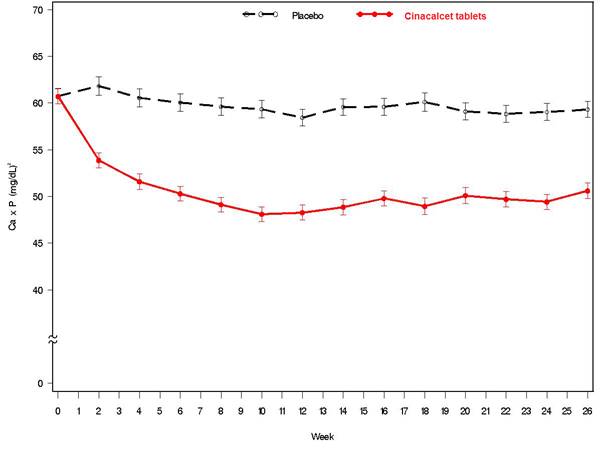
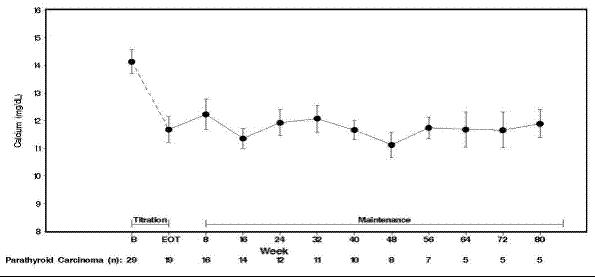
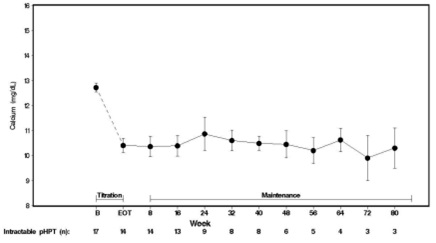
14 CLINICAL STUDIES14.1 Secondary Hyperparathyroidism in Patients with Chronic Kidney Disease on Dialysis - Three 6-month, multicenter, randomized, double-blind, placebo-controlled clinical studies of similar ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGCinacalcet hydrochloride 30 mg tablets are formulated as light-green, film-coated, oval-shaped, biconvex tablets debossed with “CL” on one side and “410” on the opposite side, packaged in bottles ...
-
17 PATIENT COUNSELING INFORMATIONHypocalcemia: Advise patients to report symptoms of hypocalcemia, including paresthesias, myalgias, muscle spasms, and seizures, to their healthcare provider [see Warnings and Precautions ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 76282-674-30 Rx Only - Cinacalcet - Hydrochloride - Tablets - 30mg* 30 Tablets - Exelan - NDC 76282-675-30 Rx Only - Cinacalcet - Hydrochloride ...
-
INGREDIENTS AND APPEARANCEProduct Information