Label: TRIMETHOPRIM tablet
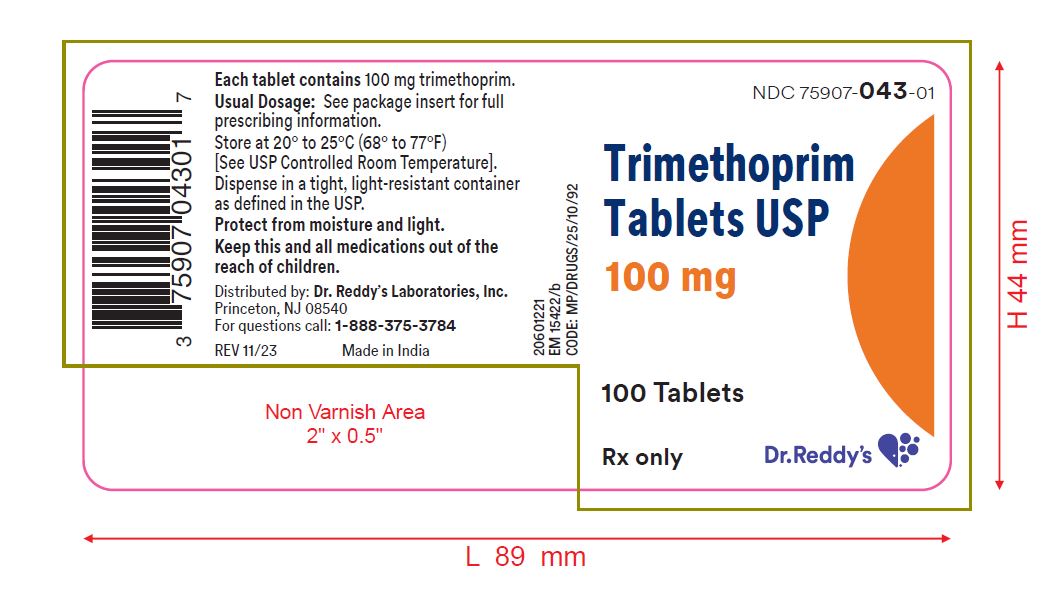
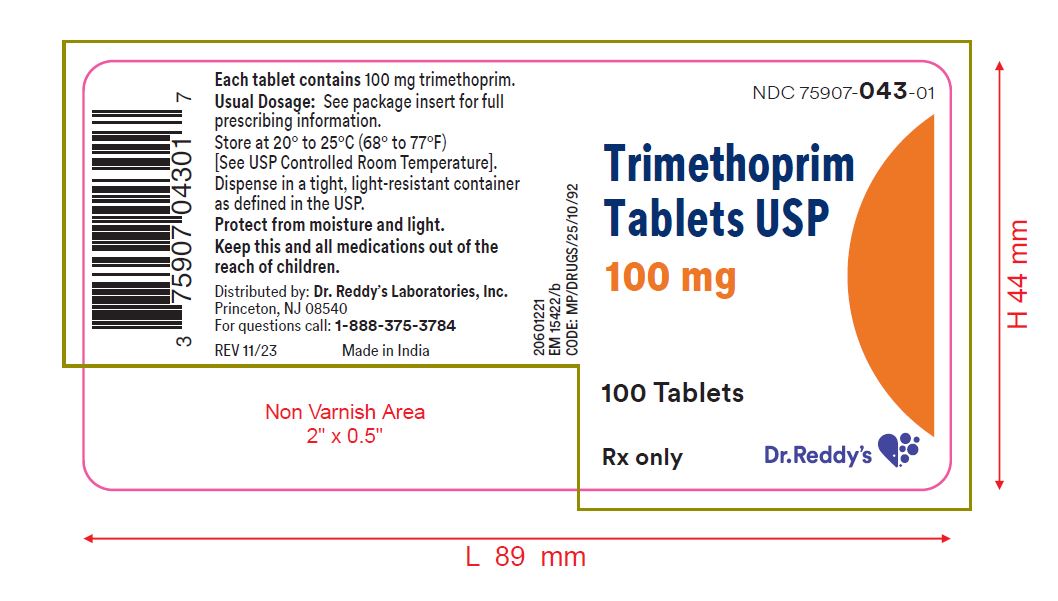
- NDC Code(s): 75907-043-01
- Packager: Dr. Reddy's Labratories Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated January 26, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONTo reduce the development of drug-resistant bacteria and maintain the effectiveness of trimethoprim tablets, USP and other antibacterial drugs, trimethoprim tablets, USP should be used only to ...
-
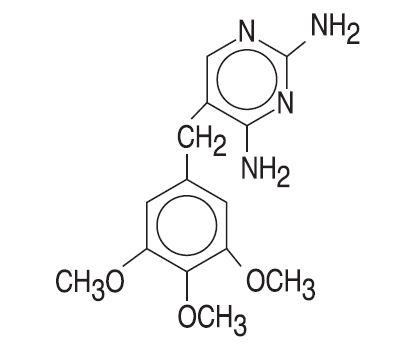
DESCRIPTIONTrimethoprim is a synthetic antibacterial available in tablet form for oral administration. Each scored white tablet contains 100 mg trimethoprim. Trimethoprim is ...
-
CLINICAL PHARMACOLOGYTrimethoprim is rapidly absorbed following oral administration. It exists in the blood as unbound, protein-bound, and metabolized forms. Ten to twenty percent of trimethoprim is metabolized ...
-
INDICATIONS AND USAGETo reduce the development of drug-resistant bacteria and maintain the effectiveness of trimethoprim tablets, USP and other antibacterial drugs, trimethoprim tablets, USP should be used only to ...
-
CONTRAINDICATIONSTrimethoprim is contraindicated in individuals hypersensitive to trimethoprim and in those with documented megaloblastic anemia due to folate deficiency.
-
WARNINGSSerious hypersensitivity reactions have been reported rarely in patients on trimethoprim therapy. Trimethoprim has been reported rarely to interfere with hematopoiesis, especially when ...
-
PRECAUTIONSGeneral - Prescribing trimethoprim tablets, USP in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and ...
-
ADVERSE REACTIONSThe adverse effects encountered most often with trimethoprim were rash and pruritus. To report SUSPECTED ADVERSE EVENTS, contact Dr. Reddy’s Laboratories Inc, at 1-888-375-3784 or FDA at ...
-
OVERDOSAGEAcute - Signs of acute overdosage with trimethoprim may appear following ingestion of 1 gram or more of the drug and include nausea, vomiting, dizziness, headaches, mental depression, confusion ...
-
DOSAGE AND ADMINISTRATIONThe usual oral adult dosage is 100 mg of trimethoprim every 12 hours or 200 mg of trimethoprim every 24 hours, each for 10 days. The use of trimethoprim in patients with a creatinine clearance of ...
-
HOW SUPPLIEDTrimethoprim tablets, USP, 100 mg: White, round, convex tablet, debossed "9", scored, "3" on one side and debossed "21 58" on the other, in bottles of 100. NDC 75907-043-01 - Store at 20° to 25°C ...
-
REFERENCES1. Brumfitt W, Pursell R. Trimethoprim-sulfamethoxazole in the treatment of bacteriuria in women. J Infect Dis. 1973;128(suppl): S657-S663. 2. Lacey RW, Simpson MHC, Fawcett C, et al. Comparison ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Dr. Reddy’s Laboratories Inc. Princeton, NJ 08540 - Made in India - Rev. 11/2023
-
PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle LabelNDC 75907-043-01 - Trimethoprim - Tablets USP - 100 mg - Rx Only - 100 Tablets - Dr. Reddy's Laboratories, Inc.
-
INGREDIENTS AND APPEARANCEProduct Information