Label: CLONIDINE patch, extended release
- NDC Code(s): 75907-023-11, 75907-023-48, 75907-024-11, 75907-024-48, view more
- Packager: Dr. Reddy's Labratories Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated February 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONProgrammed delivery in vivo of 0.1, 0.2, or 0.3 mg clonidine USP per day, for one week. Rx only
-
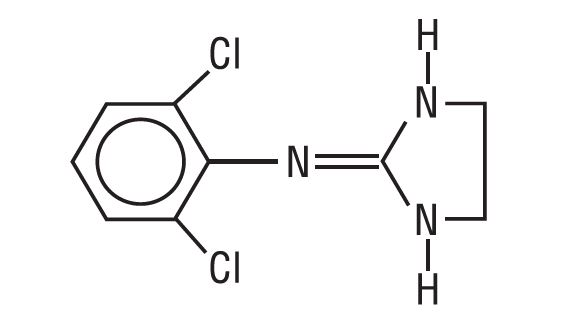
DESCRIPTIONClonidine transdermal system USP provides continuous systemic delivery of clonidine USP for 7 days at an approximately constant rate. Clonidine USP is a centrally acting alpha-agonist hypotensive ...
-
CLINICAL PHARMACOLOGYClonidine stimulates alpha-adrenoreceptors in the brain stem. This action results in reduced sympathetic outflow from the central nervous system and in decreases in peripheral resistance, renal ...
-
INDICATIONS AND USAGEClonidine transdermal system is indicated in the treatment of hypertension. It may be employed alone or concomitantly with other antihypertensive agents.
-
CONTRAINDICATIONSClonidine transdermal system should not be used in patients with known hypersensitivity to clonidine or to any other component of the therapeutic system.
-
WARNINGSWithdrawal - Patients should be instructed not to discontinue therapy without consulting their physician. Sudden cessation of clonidine treatment has, in some cases, resulted in symptoms such as ...
-
PRECAUTIONSGeneral - In patients who have developed localized contact sensitization to clonidine transdermal system, continuation of clonidine transdermal system or substitution of oral clonidine ...
-
ADVERSE REACTIONSClinical Trial Experience With Clonidine Transdermal System - Most systemic adverse effects during clonidine transdermal system therapy have been mild and have tended to diminish with continued ...
-
OVERDOSAGEHypertension may develop early and may be followed by hypotension, bradycardia, respiratory depression, hypothermia, drowsiness, decreased or absent reflexes, weakness, irritability and miosis ...
-
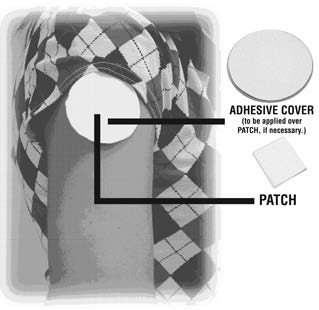
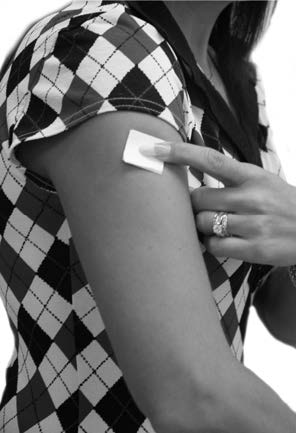
DOSAGE AND ADMINISTRATIONApply clonidine transdermal system once every 7 days to a hairless area of intact skin on the upper outer arm or chest. Each new application of clonidine transdermal system should be on a ...
-
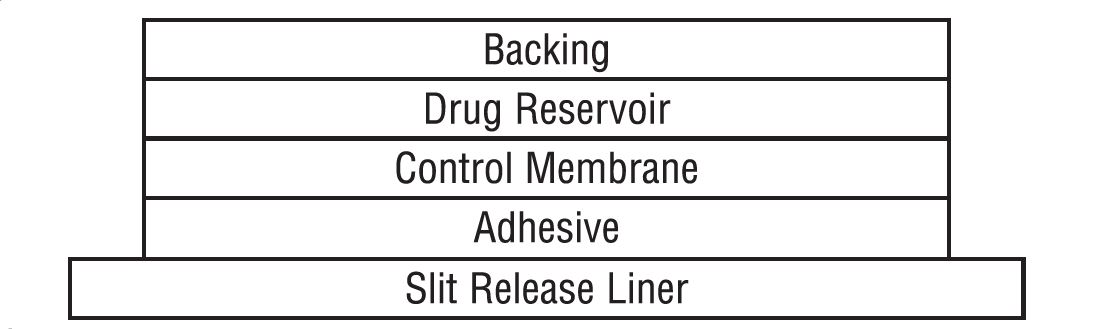
HOW SUPPLIEDClonidine transdermal system USP 0.1 mg/day, 0.2 mg/day or 0.3 mg/day are available as 4 pouched systems and 4 adhesive covers per carton. Each system is a round corner, rectangular flexible ...
-
SPL UNCLASSIFIED SECTIONDistributed by: Dr. Reddy’s Laboratories Inc. Princeton, NJ 08540 - Manufactured By: Corium Innovations, Inc. Grand Rapids, MI 49512 - Rev. 09/2023
-
PATIENT INSTRUCTIONSCLONIDINE Transdermal System USP - Rx only - (Read the following instructions carefully before using this medication. If you have any questions, please consult with your doctor.) General ...
-
PRINCIPAL DISPLAY PANEL - 0.2 mg Patch Pouch CartonNDC 75907-024-48 - Clonidine - Transdermal System USP - 0.2 mg/day - Programmed in vivo delivery of 0.2 mg per day for one week. To avoid possible burns, remove the clonidine transdermal system patch ...
-
PACKAGE LABEL PRINCIPAL DISPLAY PANEL - 0.1 mg patch pouch cartonNDC 75907-023-48 - Clonidine - Transdermal System USP - 0.2 mg/day - Programmed in vivo delivery of 0.2 mg per day for one week. To avoid possible burns, remove the clonidine transdermal system patch ...
-
PACKAGE LABEL PRINCIPLE DISPLAY PANEL - 0.3 mg patch pouch cartonNDC 75907-024-48 - Clonidine - Transdermal System USP - 0.2 mg/day - Programmed in vivo delivery of 0.2 mg per day for one week. To avoid possible burns, remove the clonidine transdermal system patch ...
-
INGREDIENTS AND APPEARANCEProduct Information