Label: OFLOXACIN tablet, coated
-
NDC Code(s):
75834-199-01,
75834-199-05,
75834-199-50,
75834-200-01, view more75834-200-05, 75834-200-50, 75834-201-01, 75834-201-05, 75834-201-50
- Packager: Nivagen Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONNivagen Pharmaceuticals, Inc. Rx only
-
BOXED WARNING
(What is this?)
WARNING: SERIOUS ADVERSE REACTIONS INCLUDING TENDINITIS, TENDON RUPTURE, PERIPHERAL NEUROPATHY, CENTRAL NERVOUS SYSTEM EFFECTS AND EXACERBATION OF MYASTHENIA GRAVIS
Fluoroquinolones, including ofloxacin, have been associated with disabling and potentially irreversible serious adverse reactions that have occurred together, including:
- Tendinitis and tendon rupture
- Peripheral neuropathy
- Central nervous system effects (see WARNINGS).
Discontinue ofloxacin immediately and avoid the use of fluoroquinolones, including ofloxacin, in patients who experience any of these serious adverse reactions (see WARNINGS).
- Fluoroquinolones, including ofloxacin, may exacerbate muscle weakness in persons with myasthenia gravis. Avoid ofloxacin in patients with a known history of myasthenia gravis (See WARNINGS).
-
Because fluoroquinolones, including ofloxacin, have been associated with serious adverse reactions (see WARNINGS), reserve ofloxacin for use in patients who have no alternative treatment options for the following indications:
- Acute exacerbation of chronic bronchitis
- Uncomplicated cystitis (see INDICATIONS and USAGE)
-
SPL UNCLASSIFIED SECTIONTo reduce the development of drug-resistant bacteria and maintain the effectiveness of ofloxacin tablets and other antibacterial drugs, ofloxacin tablets should be used only to treat or prevent ...
To reduce the development of drug-resistant bacteria and maintain the effectiveness of ofloxacin tablets and other antibacterial drugs, ofloxacin tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
Close -
DESCRIPTIONOfloxacin tablets are a synthetic broad-spectrum antimicrobial agent for oral administration. Chemically, ofloxacin, USP, a fluorinated carboxyquinolone, is the racemate ...
Ofloxacin tablets are a synthetic broad-spectrum antimicrobial agent for oral administration. Chemically, ofloxacin, USP, a fluorinated carboxyquinolone, is the racemate, (±)-9-fluoro-2,3-dihydro-3-methyl-10-(4-methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid. The chemical structure is:
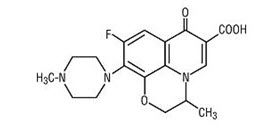
C18H20FN3O4 M.W. 361.4
Ofloxacin, USP is an off-white to pale yellow crystalline powder. The molecule exists as a zwitterion at the pH conditions in the small intestine. The relative solubility characteristics of ofloxacin, USP at room temperature, as defined by USP nomenclature, indicate that ofloxacin, USP is considered to be soluble in aqueous solutions with pH between 2 and 5. It is sparingly to slightly soluble in aqueous solutions with pH 7 (solubility falls to 4 mg/mL) and freely soluble in aqueous solutions with pH above 9. Ofloxacin, USP has the potential to form stable coordination compounds with many metal ions. This in vitro chelation potential has the following formation order: Fe+3 > Al+3 > Cu +2 > Ni +2 > Pb+2 > Zn+2 > Mg+2 > Ca +2 > Ba +2.
Ofloxacin Tablets, USP contain the following inactive ingredients: lactose monohydrate, pregelatinized maize starch, hydroxy propyl methyl cellulose, talc, magnesium stearate, polyethylene glycol, sodium starch glycolate, and titanium dioxide. Additionally, the 200 mg and 400 mg tablets contain iron oxide yellow. The imprinting ink for 200 mg, 300 mg and 400 mg strength contains FD&C blue #1, isopropyl alcohol, N-butyl alcohol, propylene glycol, shellac and titanium dioxide.
Close -
CLINICAL PHARMACOLOGYFollowing oral administration, the bioavailability of ofloxacin in the tablet formulation is approximately 98%. Maximum serum concentrations are achieved one to two hours after an oral dose ...
Following oral administration, the bioavailability of ofloxacin in the tablet formulation is approximately 98%. Maximum serum concentrations are achieved one to two hours after an oral dose. Absorption of ofloxacin after single or multiple doses of 200 to 400 mg is predictable, and the amount of drug absorbed increases proportionately with the dose. Ofloxacin has biphasic elimination. Following multiple oral doses at steady-state administration, the half-lives are approximately 4 to 5 hours and 20 to 25 hours. However, the longer half-life represents less than 5% of the total AUC. Accumulation at steady-state can be estimated using a half-life of 9 hours. The total clearance and volume of distribution are approximately similar after single or multiple doses. Elimination is mainly by renal excretion. The following are mean peak serum concentrations in healthy 70 to 80 kg male volunteers after single oral doses of 200, 300, or 400 mg of ofloxacin or after multiple oral doses of 400 mg.
Oral Dose Serum Concentration
2 Hours After Admin. (mcg/mL)Area Under the Curve
(AUC(0 to ∞)) (mcg∙h/mL)200 mg single dose 1.5 14.1 300 mg single dose 2.4 21.2 400 mg single dose 2.9 31.4 400 mg steady-state 4.6 61.0 Steady-state concentrations were attained after four oral doses, and the area under the curve (AUC) was approximately 40% higher than the AUC after single doses. Therefore, after multiple-dose administration of 200 mg and 300 mg doses, peak serum levels of 2.2 mcg/mL and 3.6 mcg/mL, respectively, are predicted at steady-state.
In vitro, approximately 32% of the drug in plasma is protein bound.
The single dose and steady-state plasma profiles of ofloxacin injection were comparable in extent of exposure (AUC) to those of ofloxacin tablets when the injectable and tablet formulations of ofloxacin were administered in equal doses (mg/mg) to the same group of subjects. The mean steady-state AUC (0 to 12) attained after the intravenous administration of 400 mg over 60 min was 43.5 mcg∙h/mL; the mean steady-state AUC (0 to 12) attained after the oral administration of 400 mg was 41.2 mcg∙h/mL (two one-sided t-test, 90% confidence interval was 103 to 109) (see following chart).
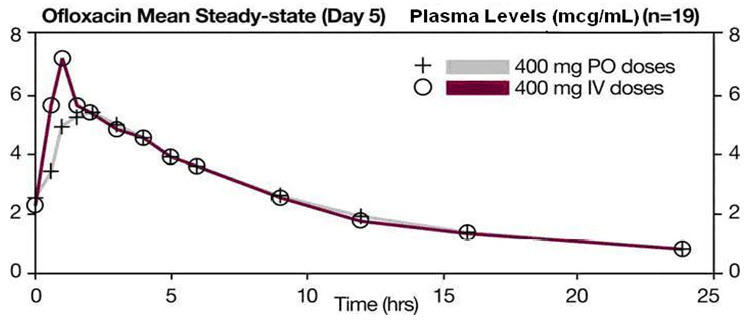
Between 0 and 6 h following the administration of a single 200 mg oral dose of ofloxacin to 12 healthy volunteers, the average urine ofloxacin concentration was approximately 220 mcg/mL. Between 12 and 24 hours after administration, the average urine ofloxacin level was approximately 34 mcg/mL.
Following oral administration of recommended therapeutic doses, ofloxacin has been detected in blister fluid, cervix, lung tissue, ovary, prostatic fluid, prostatic tissue, skin, and sputum. The mean concentration of ofloxacin in each of these various body fluids and tissues after one or more doses was 0.8 to 1.5 times the concurrent plasma level. Inadequate data are presently available on the distribution or levels of ofloxacin in the cerebrospinal fluid or brain tissue.
Ofloxacin has a pyridobenzoxazine ring that appears to decrease the extent of parent compound metabolism. Between 65% and 80% of an administered oral dose of ofloxacin is excreted unchanged via the kidneys within 48 hours of dosing. Studies indicate that less than 5% of an administered dose is recovered in the urine as the desmethyl or N-oxide metabolites. Four to eight percent of an ofloxacin dose is excreted in the feces. This indicates a small degree of biliary excretion of ofloxacin.
The administration of ofloxacin tablets with food does not affect the Cmax and AUC∞ of the drug, but Tmax is prolonged.
Clearance of ofloxacin is reduced in patients with impaired renal function (creatinine clearance rate ≤50 mL/min), and dosage adjustment is necessary (see PRECAUTIONS, General and DOSAGE AND ADMINISTRATION).
Following oral administration to healthy elderly subjects (65 to 81 years of age), maximum plasma concentrations are usually achieved one to two hours after single and multiple twice-daily doses, indicating that the rate of oral absorption is unaffected by age or gender. Mean peak plasma concentrations in elderly subjects were 9 to 21% higher than those observed in younger subjects. Gender differences in the pharmacokinetic properties of elderly subjects have been observed. Peak plasma concentrations were 114% and 54% higher in elderly females compared to elderly males following single and multiple twice-daily doses. [This interpretation was based on study results collected from two separate studies.] Plasma concentrations increase dose-dependently with the increase in doses after single oral dose and at steady state. No differences were observed in the volume of distribution values between elderly and younger subjects. As in younger subjects, elimination is mainly by renal excretion as unchanged drug in elderly subjects, although less drug is recovered from renal excretion in elderly subjects. Consistent with younger subjects, less than 5% of an administered dose was recovered in the urine as the desmethyl and N-oxide metabolites in the elderly. A longer plasma half-life of approximately 6.4 to 7.4 hours was observed in elderly subjects, compared with 4 to 5 hours for young subjects. Slower elimination of ofloxacin is observed in elderly subjects as compared with younger subjects which may be attributable to the reduced renal function and renal clearance observed in the elderly subjects. Because ofloxacin is known to be substantially excreted by the kidney, and elderly patients are more likely to have decreased renal function, dosage adjustment is necessary for elderly patients with impaired renal function as recommended for all patients. (see PRECAUTIONS, General and DOSAGE AND ADMINISTRATION).
CloseMICROBIOLOGY
Ofloxacin is a quinolone antimicrobial agent. The mechanism of action of ofloxacin and other fluoroquinolone antimicrobials involves inhibition of bacterial topoisomerase IV and DNA gyrase (both of which are type II topoisomerases), enzymes required for DNA replication, transcription, repair and recombination.
Ofloxacin has in vitro activity against a wide range of gram-negative and gram-positive microorganisms. Ofloxacin is often bactericidal at concentrations equal to or slightly greater than inhibitory concentrations.
Fluoroquinolones, including ofloxacin, differ in chemical structure and mode of action from aminoglycosides, macrolides and β-lactam antibiotics, including penicillins. Fluoroquinolones may, therefore, be active against bacteria resistant to these antimicrobials.
Resistance to ofloxacin due to spontaneous mutation in vitro is a rare occurrence (range: 10-9 to 10-11). Although cross-resistance has been observed between ofloxacin and some other fluoroquinolones, some microorganisms resistant to other fluoroquinolones may be susceptible to ofloxacin.
Ofloxacin has been shown to be active against most strains of the following microorganisms both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section:
Aerobic Gram-Positive Microorganisms
Staphylococcus aureus (methicillin-susceptible strains)
Streptococcus pneumoniae (penicillin-susceptible strains)
Streptococcus pyogenesAerobic Gram-Negative Microorganisms
Citrobacter (diversus) koseri
Enterobacter aerogenes
Escherichia coli
Haemophilus influenzae
Klebsiella pneumoniae
Neisseria gonorrhoeae
Proteus mirabilis
Pseudomonas aeruginosaAs with other drugs in this class, some strains of Pseudomonas aeruginosa may develop resistance fairly rapidly during treatment with ofloxacin.
Other Microorganisms
Chlamydia trachomatisThe following in vitro data are available, but their clinical significance is unknown.
Ofloxacin exhibits in vitro minimum inhibitory concentrations (MIC values) of 2 mcg/mL or less against most (≥ 90%) strains of the following microorganisms; however, the safety and effectiveness of ofloxacin in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled trials.
Aerobic Gram-Positive Microorganisms
Staphylococcus epidermidis (methicillin-susceptible strains)
Staphylococcus saprophyticus
Streptococcus pneumoniae (penicillin-resistant strains)Aerobic Gram-Negative Microorganisms
Acinetobacter calcoaceticus
Bordetella pertussis
Citrobacter freundii
Enterobacter cloacae
Haemophilus ducreyi
Klebsiella oxytoca
Moraxella catarrhalis
Morganella morganii
Proteus vulgaris
Providencia rettgeri
Providencia stuartii
Serratia marcescensAnaerobic Microorganisms
Clostridium perfringesOther Microorganisms
Chlamydia pneumoniae
Gardnerella vaginalis
Legionella pneumophila
Mycoplasma hominis
Mycoplasma pneumoniae
Ureaplasma urealyticumOfloxacin is not active against Treponema pallidum (see WARNINGS).
Many strains of other streptococcal species, Enterococcus species, and anaerobes are resistant to ofloxacin.
Susceptibility Tests
Dilution Techniques
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MIC values). These MIC values provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MIC values should be determined using a standardized procedure. Standardized procedures are based on a dilution method1,3 (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of ofloxacin powder. The MIC values should be interpreted according to the following criteria:
For testing Enterobacteriaceae, methicillin-susceptible Staphylococcus aureus, and Pseudomonas aeruginosa: MIC (mcg/mL) Interpretation ≤ 2 Susceptible (S) 4 Intermediate (I) ≥ 8 Resistant (R) For testing Haemophilus influenzae:* MIC (mcg/mL) Interpretation - *
- This interpretive standard is applicable only to broth microdilution susceptibility tests with Haemophilus influenzae using Haemophilus Test Medium.1,3
≤ 2 Susceptible (S) The current absence of data on resistant strains precludes defining any results other than "Susceptible." Strains yielding MIC results suggestive of a "nonsusceptible" category should be submitted to a reference laboratory for further testing.
For testing Neisseria gonorrhoeae:* MIC (mcg/mL) Interpretation - *
- These interpretive standards are applicable only to agar dilution tests using GC agar base and 1% defined growth supplement incubated in 5% CO2.
≤ 0.25 Susceptible (S) 0.5 to 1 Intermediate (I) ≥ 2 Resistant (R) For testing Streptococcus pneumoniae and Streptococcus pyogenes:* MIC (mcg/mL) Interpretation - *
- These interpretive standards are applicable only to broth microdilution susceptibility tests using cation-adjusted Mueller-Hinton broth with 2 to 5% lysed horse blood.
≤ 2 Susceptible (S) 4 Intermediate (I) ≥ 8 Resistant (R) A report of "Susceptible" indicates that the pathogen is likely to be inhibited if the antimicrobial compound in the blood reaches the concentration usually achievable. A report of "Intermediate" indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where a high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of "Resistant" indicates that the pathogen is not likely to be inhibited if the antimicrobial compound in the blood reaches the concentration usually achievable; other therapy should be selected.
Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Standard ofloxacin powder should provide the following MIC values:
Microorganism MIC Range (mcg/mL) - *
- This quality control range is applicable only to H. influenzae ATCC 49247 tested by a microdilution procedure using Haemophilus Test Medium (HTM).1,3
- †
- This quality control range is applicable only to N. gonorrhoeae ATCC 49226 tested by an agar dilution procedure using GC agar base with 1% defined growth supplement incubated in 5% CO2.
- ‡
- This quality control range is applicable only to S. pneumoniae ATCC 49619 tested by a microdilution procedure using cation-adjusted Mueller-Hinton broth with 2 to 5% lysed horse blood.
Escherichia coli ATCC 25922 0.015 to 0.12 Haemophilus influenzae ATCC 49247* 0.016 to 0.06 Neisseria gonorrhoeae ATCC 49226† 0.004 to 0.016 Pseudomonas aeruginosa ATCC 27853 1 to 8 Staphylococcus aureus ATCC 29213 0.12 to 1 Streptococcus pneumoniae ATCC 49619‡ 1 to 4 Diffusion Techniques
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure2 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 5 mcg ofloxacin to test the susceptibility of microorganisms to ofloxacin.
Reports from the laboratory providing results of the standard single-disk susceptibility test with a 5 mcg ofloxacin disk should be interpreted according to the following criteria:
For testing Enterobacteriaceae, methicillin-susceptible Staphylococcus aureus, and Pseudomonas aeruginosa: Zone Diameter (mm) Interpretation ≥ 16 Susceptible (S) 13 to 15 Intermediate (I) ≤ 12 Resistant (R) For testing Haemophilus influenzae:* Zone Diameter (mm) Interpretation - *
- This zone diameter standard is applicable only to disk diffusion tests with Haemophilus influenzae using Haemophilus Test Medium (HTM)2 incubated in 5% CO2.
≥ 16 Susceptible (S) The current absence of data on resistant strains precludes defining any results other than "Susceptible." Strains yielding zone diameter results suggestive of a "nonsusceptible" category should be submitted to a reference laboratory for further testing.
For testing Neisseria gonorrhoeae:* Zone Diameter (mm) Interpretation - *
- These zone diameter standards are applicable only to disk diffusion tests using GC agar base and 1% defined growth supplement incubated in 5% CO2.
≥ 31 Susceptible (S) 25 to 30 Intermediate (I) ≤ 24 Resistant (R) For testing Streptococcus pneumoniae and Streptococcus pyogenes:* Zone Diameter (mm) Interpretation - *
- These zone diameter standards are applicable only to disk diffusion tests performed using Mueller-Hinton agar supplemented with 5% defibrinated sheep blood and incubated in 5% CO2.
≥ 16 Susceptible (S) 13 to 15 Intermediate (I) ≤ 12 Resistant (R) Interpretation should be as stated above for results using dilution techniques. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for ofloxacin.
As with standardized dilution techniques, diffusion methods require the use of laboratory control microorganisms that are used to control the technical aspects of the laboratory procedures. For the diffusion technique, the 5 mcg ofloxacin disk should provide the following zone diameters in these laboratory quality control strains:
Microorganism Zone Diameter (mm) - *
- This quality control range is applicable only to H. influenzae ATCC 49247 tested by a disk diffusion procedure using Haemophilus Test Medium (HTM)2 incubated in 5% CO2.
- †
- This quality control range is applicable only to N. gonorrhoeae ATCC 49226 tested by a disk diffusion procedure using GC agar base with 1% defined growth supplement incubated in 5% CO2.
- ‡
- This quality control range is applicable only to S. pneumoniae ATCC 49619 tested by a disk diffusion procedure using Mueller-Hinton agar supplemented with 5% defibrinated sheep blood and incubated in 5% CO2.
Escherichia coli ATCC 25922 29 to 33 Haemophilus influenzae ATCC 49247* 31 to 40 Neisseria gonorrhoeae ATCC 49226† 43 to 51 Pseudomonas aeruginosa ATCC 27853 17 to 21 Staphylococcus aureus ATCC 25923 24 to 28 Streptococcus pneumoniae ATCC 49619‡ 16 to 21 -
INDICATIONS AND USAGETo reduce the development of drug-resistant bacteria and maintain the effectiveness of ofloxacin tablets, USP and other antibacterial drugs, ofloxacin tablets, USP should be used only to treat or ...
To reduce the development of drug-resistant bacteria and maintain the effectiveness of ofloxacin tablets, USP and other antibacterial drugs, ofloxacin tablets, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Ofloxacin tablets, USP are indicated for the treatment of adults with mild to moderate infections (unless otherwise indicated) caused by susceptible strains of the designated microorganisms in the infections listed below. Please see DOSAGE AND ADMINISTRATION for specific recommendations.
Acute Bacterial Exacerbations of Chronic Bronchitis (ABECB) due to Haemophilus influenzae or Streptococcus pneumoniae.
Because fluoroquinolones, including ofloxacin, have been associated with serious adverse reactions (see Warnings), -and for some patients ABECB is self-limiting, reserve ofloxacin for treatment of ABECB in patients who have no alternative treatment options.
Community-Acquired Pneumonia due to Haemophilus influenzae or Streptococcus pneumoniae.
Uncomplicated Skin and Skin Structure Infections due to methicillin-susceptible Staphylococcus aureus, Streptococcus pyogenes, or Proteus mirabilis.
Acute, Uncomplicated Urethral and Cervical Gonorrhea due to Neisseria gonorrhoeae (see WARNINGS).
Nongonococcal Urethritis and Cervicitis due to Chlamydia trachomatis (see WARNINGS).
Mixed Infections of the Urethra and Cervix due to Chlamydia trachomatis and Neisseria gonorrhoeae (see WARNINGS).
Acute Pelvic Inflammatory Disease (including severe infection) due to Chlamydia trachomatis and/or Neisseria gonorrhoeae (see WARNINGS).
NOTE: If anaerobic microorganisms are suspected of contributing to the infection, appropriate therapy for anaerobic pathogens should be administered.
Uncomplicated Cystitis due to Citrobacter diversus, Enterobacter aerogenes, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, or Pseudomonas aeruginosa.
Because fluoroquinolones, including ofloxacin, have been associated with serious adverse reactions (see WARNINGS), and for some patients uncomplicated cystitis is self-limiting, reserve ofloxacin for treatment of uncomplicated cystitis in patients who have no alternative treatment options.
Complicated Urinary Tract Infections due to Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Citrobacter diversus,1 or Pseudomonas aeruginosa.1
Prostatitis due to Escherichia coli.
Appropriate culture and susceptibility tests should be performed before treatment in order to isolate and identify organisms causing the infection and to determine their susceptibility to ofloxacin, USP. Therapy with ofloxacin, USP may be initiated before results of these tests are known; once results become available, appropriate therapy should be continued.
As with other drugs in this class, some strains of Pseudomonas aeruginosa may develop resistance fairly rapidly during treatment with ofloxacin, USP. Culture and susceptibility testing performed periodically during therapy will provide information not only on the therapeutic effect of the antimicrobial agent but also on the possible emergence of bacterial resistance.
- 1
- = Although treatment of infections due to this organism in this organ system demonstrated a clinically significant outcome, efficacy was studied in fewer than 10 patients.
-
CONTRAINDICATIONSOfloxacin tablets are contraindicated in persons with a history of hypersensitivity associated with the use of ofloxacin or any member of the quinolone group of antimicrobial agents.
Ofloxacin tablets are contraindicated in persons with a history of hypersensitivity associated with the use of ofloxacin or any member of the quinolone group of antimicrobial agents.
Close -
WARNINGSDisabling and Potentially Irreversible Serious Adverse Reactions Including Tendinitis and Tendon Rupture, Peripheral Neuropathy, and Central Nervous System Effects - Fluoroquinolones, including ...Close
Disabling and Potentially Irreversible Serious Adverse Reactions Including Tendinitis and Tendon Rupture, Peripheral Neuropathy, and Central Nervous System Effects
Fluoroquinolones, including ofloxacin, have been associated with disabling and potentially irreversible serious adverse reactions from different body systems that can occur together in the same patient. Commonly seen adverse reactions include tendinitis, tendon rupture, arthralgia, myalgia, peripheral neuropathy, and central nervous system effects (hallucinations, anxiety, depression, insomnia, severe headaches, and confusion). These reactions can occur within hours to weeks after starting ofloxacin. Patients of any age or without pre-existing risk factors have experienced these adverse reactions (see Warnings)
Discontinue ofloxacin immediately at the first signs or symptoms of any serious adverse reaction. In addition, avoid the use of fluoroquinolones, including ofloxacin, in patients who have experienced any of these serious adverse reactions associated with fluoroquinolones.
Tendinitis and Tendon Rupture
Fluoroquinolones, including ofloxacin, have been associated with an increased risk of tendinitis and tendon rupture in all ages. This adverse reaction most frequently involves the Achilles tendon, and rupture of the Achilles tendon and has been reported with the rotator cuff (the shoulder), the hand, the biceps, the thumb, and other tendons. Tendinitis or tendon rupture can occur within hours or days of starting ofloxacin, or as long as several months after completion of fluoroquinolone therapy. Tendinitis and tendon rupture can occur bilaterally.
The risk of developing fluoroquinolone-associated tendinitis and tendon rupture is increased in patients over 60 years of age, in those taking corticosteroid drugs, and in patients with kidney, heart or lung transplants. Other factors that may independently increase the risk of tendon rupture include strenuous physical activity, renal failure, and previous tendon disorders such as rheumatoid arthritis. Tendinitis and tendon rupture have been reported in patients taking fluoroquinolones who do not have the above risk factors. Discontinue ofloxacin immediately if the patient experiences pain, swelling, inflammation or rupture of a tendon. Avoid fluoroquinolones, including ofloxacin, in patients who have a history of tendon disorders or have experienced tendinitis or tendon rupture (see Adverse Reactions). Patients should be advised to rest at the first sign of tendinitis or tendon rupture, and to contact their healthcare provider regarding changing to a non-quinolone antimicrobial drug.
Peripheral Neuropathy
Fluoroquinolones, including ofloxacin, have been associated with an increased risk of peripheral neuropathy. Cases of sensory or sensorimotor axonal polyneuropathy affecting small and/or large axons resulting in paresthesias, hypoesthesias, dysesthesias and weakness have been reported in patients receiving fluoroquinolones, including ofloxacin. Symptoms may occur soon after initiation of norfloxacin and may be irreversible in some patients (see WARNINGS).
Discontinue ofloxacin immediately if the patient experiences symptoms of peripheral neuropathy including pain, burning, tingling, numbness, and/or weakness, or other alterations in sensations including light touch, pain, temperature, position sense and vibratory sensation, and/or motor strength in order to minimize the development of an irreversible condition. Avoid fluoroquinolones, including ofloxacin, in patients who have previously experienced peripheral neuropathy (see Adverse Reactions)
Central Nervous System Effects
Psychiatric Adverse Reactions
Fluoroquinolones, including ofloxacin, have been associated with an increased risk of psychiatric adverse reactions toxic psychoses or hallucinations; agitation; delirium, confusion, disorientation, or disturbances in attention; nervousness or restlessness, memory impairment. If these reactions occur in patients receiving ofloxacin, the drug should be discontinued and appropriate measures instituted.
Central Nervous System Adverse Reactions
Fluoroquinolones have been associated with an increased risk of seizures (convulsions), increased intracranial pressure (pseudotumor cerebri), lightheadedness, or tremors. The effects of ofloxacin on brain function or on the electrical activity of the brain have not been tested. Therefore, until more information becomes available, ofloxacin, like all other quinolones, should be used with caution in patients with known or suspected CNS disorders, such as severe cerebral arteriosclerosis, epilepsy, and other factors which predispose to seizures (see ADVERSE REACTIONS).
Exacerbation of Myasthenia Gravis
Fluoroquinolones, including ofloxacin, have neuromuscular blocking activity and may exacerbate muscle weakness in persons with myasthenia gravis. Postmarketing serious adverse events, including deaths and requirement for ventilatory support, have been associated with fluoroquinolone use in persons with myasthenia gravis. Avoid ofloxacin in patients with known history of myasthenia gravis (see PRECAUTIONS, Information for Patients and ADVERSE REACTIONS, Postmarketing Adverse Events).
THE SAFETY AND EFFICACY OF OFLOXACIN IN PEDIATRIC PATIENTS AND ADOLESCENTS (UNDER THE AGE OF 18 YEARS), PREGNANT WOMEN, AND LACTATING WOMEN HAVE NOT BEEN ESTABLISHED (see PRECAUTIONS, Pediatric Use, Pregnancy, and Nursing Mothers Subsections).
In the immature rat, the oral administration of ofloxacin at 5 to 16 times the recommended maximum human dose based on mg/kg or 1 to 3 times based on mg/m2 increased the incidence and severity of osteochondrosis. The lesions did not regress after 13 weeks of drug withdrawal. Other quinolones also produce similar erosions in the weight-bearing joints and other signs of arthropathy in immature animals of various species (see ANIMAL PHARMACOLOGY).
Hypersensitivity Reactions
Serious and occasionally fatal hypersensitivity and/or anaphylactic reactions have been reported in patients receiving therapy with quinolones, including ofloxacin. These reactions often occur following the first dose. Some reactions have been accompanied by cardiovascular collapse, hypotension/shock, seizure, loss of consciousness, tingling, angioedema (including tongue, laryngeal, throat, or facial edema/swelling), airway obstruction (including bronchospasm, shortness of breath, and acute respiratory distress), dyspnea, urticaria, itching, and other serious skin reactions. This drug should be discontinued immediately at the first appearance of a skin rash or any other sign of hypersensitivity. Serious acute hypersensitivity reactions may require treatment with epinephrine and other resuscitative measures, including oxygen, intravenous fluids, antihistamines, corticosteroids, pressor amines, and airway management, as clinically indicated (see PRECAUTIONS and ADVERSE REACTIONS).
Other serious and sometimes fatal events, some due to hypersensitivity, and some due to uncertain etiology, have been reported rarely in patients receiving therapy with quinolones, including ofloxacin. These events may be severe and generally occur following the administration of multiple doses. Clinical manifestations may include one or more of the following:
- fever, rash, or severe dermatologic reactions (e.g., toxic epidermal necrolysis, Stevens-Johnson syndrome);
- vasculitis; arthralgia; myalgia; serum sickness;
- allergic pneumonitis;
- interstitial nephritis; acute renal insufficiency or failure;
- hepatitis; jaundice; acute hepatic necrosis or failure;
- anemia, including hemolytic and aplastic; thrombocytopenia, including thrombotic thrombocytopenic purpura; leukopenia; agranulocytosis; pancytopenia; and/or other hematologic abnormalities.
The drug should be discontinued immediately at the first appearance of skin rash, jaundice, or any other sign of hypersensitivity and supportive measures instituted (see PRECAUTIONS, Information for Patients and ADVERSE REACTIONS).
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including ofloxacin tablets, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated (see ADVERSE REACTIONS).
Risk of Aortic Aneurysm and Dissection
Fluoroquinolones, including ofloxacin, have been associated with aortic aneurysm and dissection. Findings from epidemiologic studies show a consistently increased risk of hospitalization for aortic aneurysm or dissection within two months following use of a fluoroquinolone antibacterial drug. The annual estimated background risk of aortic aneurysm is as high as approximately 300 aortic aneurysm events per 100,000 persons at the highest risk (e.g., age greater than 85 years). The evidence shows the potential for a 2-fold increased risk over the background risk following fluoroquinolone exposure and was based on a small number of cases, mostly in older patients. The cause for the risk of aortic aneurysm or dissection has not been identified, but the available data suggest that use of fluoroquinolones may contribute in the short term to aneurysm progression. In patients with a known aortic aneurysm or patients who are at greater risk for aortic aneurysms, reserve ofloxacin, for use only when there are no alternative antibacterial treatments available.
Ofloxacin has not been shown to be effective in the treatment of syphilis.
Fluoroquinolones have been associated with disturbances of blood glucose, including symptomatic hyperglycemia and hypoglycemia, usually in diabetic patients receiving concomitant treatment with an oral hypoglycemic agent (e.g., glyburide) or with insulin. In these patients, careful monitoring of blood glucose is recommended. Severe cases of hypoglycemia resulting in coma or death have been reported. If a hypoglycemic reaction occurs in a patient being treated with ofloxacin, discontinue ofloxacin and initiate appropriate therapy immediately.
Antimicrobial agents used in high doses for short periods of time to treat gonorrhea may mask or delay the symptoms of incubating syphilis. All patients with gonorrhea should have a serologic test for syphilis at the time of diagnosis. Patients treated with ofloxacin for gonorrhea should have a follow-up serologic test for syphilis after three months and, if positive, treatment with an appropriate antimicrobial should be instituted.
-
PRECAUTIONSGeneral - Prescribing ofloxacin tablets in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and ...
General
Prescribing ofloxacin tablets in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Adequate hydration of patients receiving ofloxacin should be maintained to prevent the formation of a highly concentrated urine.
Administer ofloxacin with caution in the presence of renal or hepatic insufficiency/impairment. In patients with known or suspected renal or hepatic insufficiency/impairment, careful clinical observation and appropriate laboratory studies should be performed prior to and during therapy since elimination of ofloxacin may be reduced. In patients with impaired renal function (creatinine clearance ≤ 50 mg/mL), alteration of the dosage regimen is necessary (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION).
Moderate to severe photosensitivity/phototoxicity reactions, the latter of which may manifest as exaggerated sunburn reactions (e.g., burning, erythema, exudation, vesicles, blistering, edema) involving areas exposed to light (typically the face, "V" area of the neck, extensor surfaces of the forearms, dorsa of the hands), can be associated with the use of quinolones after sun or UV light exposure. Therefore, excessive exposure to these sources of light should be avoided. Drug therapy should be discontinued if photosensitivity/phototoxicity occurs (See ADVERSE REACTIONS, Postmarketing Adverse Events).
As with other quinolones, ofloxacin should be used with caution in any patient with a known or suspected CNS disorder that may predispose to seizures or lower the seizure threshold (e.g., severe cerebral arteriosclerosis, epilepsy) or in the presence of other risk factors that may predispose to seizures or lower the seizure threshold (e.g., certain drug therapy, renal dysfunction) (see WARNINGS and Drug Interactions).
A possible interaction between oral hypoglycemic drugs (e.g., glyburide/glibenclamide) or with insulin and fluoroquinolone antimicrobial agents have been reported resulting in a potentiation of the hypoglycemic action of these drugs, sometimes resulting in coma or death. The mechanism for this interaction is not known. If a hypoglycemic reaction occurs in a patient being treated with ofloxacin, discontinue ofloxacin immediately and consult a physician (see Drug Interactions and ADVERSE REACTIONS).
As with any potent drug, periodic assessment of organ system functions, including renal, hepatic, and hematopoietic, is advisable during prolonged therapy (see WARNINGS and ADVERSE REACTIONS).
Torsade de Pointes
Some quinolones, including ofloxacin, have been associated with prolongation of the QT interval on the electrocardiogram and infrequent cases of arrhythmia. Rare cases of torsade de pointes have been spontaneously reported during postmarketing surveillance in patients receiving quinolones, including ofloxacin. Ofloxacin should be avoided in patients with known prolongation of the QT interval, patients with uncorrected hypokalemia, and patients receiving Class IA (quinidine, procainamide), or Class III (amiodarone, sotalol) antiarrhythmic agents.
Information for Patients
Advise the patient to read the FDA-approved patient labeling (Medication Guide)
Serious Adverse Reactions
Advise patients to stop taking ofloxacin if they experience an adverse reaction and to call their healthcare provider for advice on completing the full course of treatment with another antibacterial drug.
Inform patients of the following serious adverse reactions that have been associated with NOROXIN or other fluoroquinolone use:
- Disabling and potentially irreversible serious adverse reactions that may occur together: Inform patients that disabling and potentially irreversible serious adverse reactions, including tendinitis and tendon rupture, peripheral neuropathies, and central nervous system effects, have been associated with use of ofloxacin and may occur together in the same patient. Inform patients to stop taking ofloxacin immediately if they experience an adverse reaction and to call their healthcare provider.
- Tendon Disorders: instruct patients to contact their healthcare provider if they experience pain, swelling, or inflammation of a tendon, or weakness or inability to use one of their joints; rest and refrain from exercise; and discontinue ofloxacin treatment. The risk of severe tendon disorders with fluoroquinolones is higher in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants.
- Peripheral Neuropathies: Inform patients that peripheral neuropathies have been associated with the use of ofloxacin, that symptoms may occur soon after initiation of therapy and may be irreversible. If symptoms of peripheral neuropathy including pain, burning, tingling, numbness, and/or weakness develop, patients should immediately discontinue ofloxacin and contact their physicians.
- Central nervous system effects (for example, convulsions, dizziness, lightheadedness, increased intracranial pressure): Inform patients that convulsions have been reported in patients receiving fluoroquinolones, including ofloxacin. Instruct patients to notify their physician before taking this drug if they have a history of convulsions. Inform patients that they should know how they react to ofloxacin before they operate an automobile or machinery or engage in other activities requiring mental alertness and coordination. Instruct patients to notify their physician if persistent headache with or without blurred vision occurs.
- Myasthenia gravis: inform patients that fluoroquinolones like ofloxacin may cause worsening of myasthenia gravis symptoms, including muscle weakness and breathing problems. Patients should call their healthcare provider right away if you have any worsening muscle weakness or breathing problems.
- Hypersensitivity Reactions: Inform patients that ofloxacin can cause hypersensitivity reactions, even following a single dose, and to discontinue the drug at the first sign of a skin rash, hives or other skin reactions, a rapid heartbeat, difficulty in swallowing or breathing, any swelling suggesting angioedema (for example, swelling of the lips, tongue, face, tightness of the throat, hoarseness), or other symptoms of an allergic reaction.
- Hepatotoxicity: Inform patients that severe hepatotoxicity (including acute hepatitis and fatal events) has been reported in patients taking ofloxacin. Instruct patients to inform their physician if they experience any signs or symptoms of liver injury including: loss of appetite, nausea, vomiting, fever, weakness, tiredness, right upper quadrant tenderness, itching, yellowing of the skin and eyes, light colored bowel movements or dark colored urine.
- Diarrhea: Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, instruct patients to contact their physician as soon as possible.
- Photosensitivity/Phototoxicity: Inform patients that photosensitivity/phototoxicity has been reported in patients receiving fluoroquinolones. Patients should minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while taking quinolones. If patients need to be outdoors while using quinolones, they should wear loose-fitting clothes that protect skin from sun exposure and discuss other sun protection measures with their physician. If a sunburn-like reaction or skin eruption occurs, patients should contact their physician.
Other Information
Patients should be advised:
- to drink fluids liberally.
- that mineral supplements, vitamins with iron or minerals, calcium-, aluminum- or magnesium-based antacids, sucralfate or didanosine chewable/buffered tablets or the pediatric powder for oral solution should not be taken within the two-hour period before or within the two-hour period after taking ofloxacin (see Drug Interactions)
- that ofloxacin can be taken without regard to meals
- Patients should be counseled that antibacterial drugs including ofloxacin tablets should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When ofloxacin tablets are prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by ofloxacin tablets or other antibacterial drugs in the future.
- that if they are diabetic and are being treated with insulin or an oral hypoglycemic drug, to discontinue ofloxacin immediately if a hypoglycemic reaction occurs and consult a physician (see PRECAUTIONS, General and Drug Interactions);
- that convulsions have been reported in patients taking quinolones, including ofloxacin, and to notify their physician before taking this drug if there is a history of this condition;
- to inform their physician of any personal or family history of QTc prolongation or proarrhythmic conditions such as hypokalemia, bradycardia, or recent myocardial ischemia; if they are taking any class IA (quinidine, procainamide), or class III (amiodarone, sotalol) antiarrhythmic agents. Patients should notify their physicians if they have any symptoms of prolongation of the QTc interval including prolonged heart palpitations or a loss of consciousness.
- Inform patients who have or are at risk for an aortic aneurysm that fluoroquinolones, including ofloxacin, have been associated with a 2-fold increased risk of hospitalization for aortic aneurysm and dissection. Inform patients to seek emergency medical care if they experience sudden chest, stomach, or back pain.
Drug Interactions
Antacids, Sucralfate, Metal Cations, Multivitamins
Quinolones form chelates with alkaline earth and transition metal cations. Administration of quinolones with antacids containing calcium, magnesium, or aluminum, with sucralfate, with divalent or trivalent cations such as iron, or with multivitamins containing zinc or with didanosine, chewable/buffered tablets or the pediatric powder for oral solution may substantially interfere with the absorption of quinolones resulting in systemic levels considerably lower than desired. These agents should not be taken within the two-hour period before or within the two-hour period after ofloxacin administration (see DOSAGE AND ADMINISTRATION).
Cimetidine
Cimetidine has demonstrated interference with the elimination of some quinolones. This interference has resulted in significant increases in half-life and AUC of some quinolones. The potential for interaction between ofloxacin and cimetidine has not been studied.
Cyclosporine
Elevated serum levels of cyclosporine have been reported with concomitant use of cyclosporine with some other quinolones. The potential for interaction between ofloxacin and cyclosporine has not been studied.
Drugs Metabolized by Cytochrome P450 Enzymes
Most quinolone antimicrobial drugs inhibit cytochrome P450 enzyme activity. This may result in a prolonged half-life for some drugs that are also metabolized by this system (e.g., cyclosporine, theophylline/methylxanthines, warfarin) when coadministered with quinolones. The extent of this inhibition varies among different quinolones. (see other Drug Interactions).
Non Steroidal Anti-Inflammatory Drugs
The concomitant administration of a non-steroidal anti-inflammatory drug with a quinolone, including ofloxacin, may increase the risk of CNS stimulation and convulsive seizures (see WARNINGS and PRECAUTIONS, General).
Probenecid
The concomitant use of probenecid with certain other quinolones has been reported to affect renal tubular secretion. The effect of probenecid on the elimination of ofloxacin has not been studied.
Theophylline
Steady-state theophylline levels may increase when ofloxacin and theophylline are administered concurrently. As with other quinolones, concomitant administration of ofloxacin may prolong the half-life of theophylline, elevate serum theophylline levels, and increase the risk of theophylline-related adverse reactions. Theophylline levels should be closely monitored and theophylline dosage adjustments made, if appropriate, when ofloxacin is coadministered. Adverse reactions (including seizures) may occur with or without an elevation in the serum theophylline level. (see WARNINGS and PRECAUTIONS, General)
Warfarin
Some quinolones have been reported to enhance the effects of the oral anticoagulant warfarin or its derivatives. Therefore, if a quinolone antimicrobial is administered concomitantly with warfarin or its derivatives, the prothrombin time or other suitable coagulation test should be closely monitored.
Antidiabetic Agents (e.g., Insulin, Glyburide/Glibenclamide)
Since disturbances of blood glucose, including hyperglycemia and hypoglycemia, have been reported in patients treated concurrently with quinolones and an antidiabetic agent, careful monitoring of blood glucose is recommended when these agents are used concomitantly (see PRECAUTIONS, General and Information for Patients).
Interaction With Laboratory or Diagnostic Testing
Some quinolones, including ofloxacin, may produce false-positive urine screening results for opiates using commercially available immunoassay kits. Confirmation of positive opiate screens by more specific methods may be necessary.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies to determine the carcinogenic potential of ofloxacin have not been conducted.
Ofloxacin was not mutagenic in the Ames bacterial test, in vitro and in vivo cytogenetic assay, sister chromatid exchange (Chinese Hamster and Human Cell Lines), unscheduled DNA Repair (UDS) using human fibroblasts, dominant lethal assays, or mouse micronucleus assay. Ofloxacin was positive in the UDS test using rat hepatocytes and Mouse Lymphoma Assay.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Ofloxacin has not been shown to have any teratogenic effects at oral doses as high as 810 mg/kg/day (11 times the recommended maximum human dose based on mg/m2 or 50 times based on mg/kg) and 160 mg/kg/day (4 times the recommended maximum human dose based on mg/m2 or 10 times based on mg/kg) when administered to pregnant rats and rabbits, respectively. Additional studies in rats with oral doses up to 360 mg/kg/day (5 times the recommended maximum human dose based on mg/m2 or 23 times based on mg/kg) demonstrated no adverse effect on late fetal development, labor, delivery, lactation, neonatal viability, or growth of the newborn. Doses equivalent to 50 and 10 times the recommended maximum human dose of ofloxacin (based on mg/kg) were fetotoxic (i.e., decreased fetal body weight and increased fetal mortality) in rats and rabbits, respectively. Minor skeletal variations were reported in rats receiving doses of 810 mg/kg/day, which is more than 10 times higher than the recommended maximum human dose based on mg/m2.
There are, however, no adequate and well-controlled studies in pregnant women. Ofloxacin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus (see WARNINGS).
Nursing Mothers
In lactating females, a single oral 200 mg dose of ofloxacin resulted in concentrations of ofloxacin in milk that were similar to those found in plasma. Because of the potential for serious adverse reactions from ofloxacin in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother (see WARNINGS and ADVERSE REACTIONS).
Pediatric Use
Safety and effectiveness in pediatric patients and adolescents below the age of 18 years have not been established. Ofloxacin causes arthropathy (arthrosis) and osteochondrosis in juvenile animals of several species (see WARNINGS).
CloseGeriatric Use
Geriatric patients are at increased risk for developing severe tendon disorders including tendon rupture when being treated with a fluoroquinolone such as ofloxacin. This risk is further increased in patients receiving concomitant corticosteroid therapy. Tendinitis or tendon rupture can involve the Achilles, hand, shoulder, or other tendon sites and can occur during or after completion of therapy; cases occurring up to several months after fluoroquinolone treatment have been reported. Caution should be used when prescribing ofloxacin to elderly patients especially those on corticosteroids. Patients should be informed of this potential side effect and advised to discontinue ofloxacin and contact their healthcare provider if any symptoms of tendinitis or tendon rupture occur (See Boxed Warning; WARNINGS; and ADVERSE REACTIONS, Postmarketing Adverse Event Reports).
In phase 2/3 clinical trials with ofloxacin, 688 patients (14.2%) were ≥ 65 years of age. Of these, 436 patients (9%) were between the ages of 65 and 74 and 252 patients (5.2%) were 75 years or older. There was no apparent difference in the frequency or severity of adverse reactions in elderly adults compared with younger adults. The pharmacokinetic properties of ofloxacin in elderly subjects are similar to those in younger subjects. Drug absorption appears to be unaffected by age. Dosage adjustment is necessary for elderly patients with impaired renal function (creatinine clearance rate ≤ 50 mL/min) due to reduced clearance of ofloxacin. In comparative studies, the frequency and severity of most drug-related nervous system events in patients ≥ 65 years of age were comparable for ofloxacin and control drugs. The only differences identified were an increase in reports of insomnia (3.9% vs. 1.5%) and headache (4.7% vs. 1.8%) with ofloxacin. It is important to note that these geriatric safety data are extracted from 44 comparative studies where the adverse reaction information from 20 different controls (other antibiotics or placebo) were pooled for comparison with ofloxacin. The clinical significance of such a comparison is not clear (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION).
Elderly patients may be more sensitive to drug-associated effects on the QT interval. Therefore, precaution should be taken when using ofloxacin with concomitant drugs that can result in prolongation of the QT interval (e.g. Class IA or Class III antiarrhythmics) or in patients with risk factors for torsade de pointes (e.g. known QT prolongation, uncorrected hypokalemia) (see PRECAUTIONS, General, Torsade de Pointes).
Elderly patients are at greater risk for aortic aneurysm and dissection. A two-fold increased risk of aortic aneurysm and dissection has been reported following use of a fluoroquinolone, including ofloxacin [see Warnings].
-
ADVERSE REACTIONSThe following is a compilation of the data for ofloxacin based on clinical experience with both the oral and intravenous formulations. The incidence of drug-related adverse reactions in patients ...
The following is a compilation of the data for ofloxacin based on clinical experience with both the oral and intravenous formulations. The incidence of drug-related adverse reactions in patients during Phase 2 and 3 clinical trials was 11%. Among patients receiving multiple-dose therapy, 4% discontinued ofloxacin due to adverse experiences
In clinical trials, the following events were considered likely to be drug-related in patients receiving multiple doses of ofloxacin:
nausea 3%, insomnia 3%, headache 1%, dizziness 1%, diarrhea 1%, vomiting 1%, rash 1%, pruritus 1%, external genital pruritus in women 1%, vaginitis 1%, dysgeusia 1%.
In clinical trials, the most frequently reported adverse events, regardless of relationship to drug, were:
nausea 10%, headache 9%, insomnia 7%, external genital pruritus in women 6%, dizziness 5%, vaginitis 5%, diarrhea 4%, vomiting 4%.
In clinical trials, the following events, regardless of relationship to drug, occurred in 1 to 3% of patients:
abdominal pain and cramps, chest pain, decreased appetite, dry mouth, dysgeusia, fatigue, flatulence, gastrointestinal distress, nervousness, pharyngitis, pruritus, fever, rash, sleep disorders, somnolence, trunk pain, vaginal discharge, visual disturbances, and constipation.
Additional events, occurring in clinical trials at a rate of less than 1%, regardless of relationship to drug, were:
Body as a Whole: asthenia, chills, malaise, extremity pain, pain, epistaxis Cardiovascular System: cardiac arrest, edema, hypertension, hypotension, palpitations, vasodilation Gastrointestinal System: dyspepsia Genital/Reproductive System: burning, irritation, pain and rash of the female genitalia; dysmenorrhea; menorrhagia; metrorrhagia Musculoskeletal System: arthralgia, myalgia Nervous System: seizures, anxiety, cognitive change, depression, dream abnormality, euphoria, hallucinations, paresthesia, syncope, vertigo, tremor, confusion Nutritional/Metabolic: thirst, weight loss Respiratory System: respiratory arrest, cough, rhinorrhea Skin/Hypersensitivity: angioedema, diaphoresis, urticaria, vasculitis Special Senses: decreased hearing acuity, tinnitus, photophobia Urinary System: dysuria, urinary frequency, urinary retention The following laboratory abnormalities appeared in ≥ 1% of patients receiving multiple doses of ofloxacin. It is not known whether these abnormalities were caused by the drug or the underlying conditions being treated.
Hematopoietic: anemia, leukopenia, leukocytosis, neutropenia, neutrophilia, increased band forms, lymphocytopenia, eosinophilia, lymphocytosis, thrombocytopenia, thrombocytosis, elevated ESR Hepatic: elevated: alkaline phosphatase, AST (SGOT), ALT (SGPT) Serum Chemistry: hyperglycemia, hypoglycemia, elevated creatinine, elevated BUN Urinary: glucosuria, proteinuria, alkalinuria, hyposthenuria, hematuria, pyuria ClosePostmarketing Adverse Events
Additional adverse events, regardless of relationship to drug, reported from worldwide marketing experience with quinolones, including ofloxacin:
Clinical
Cardiovascular System: cerebral thrombosis, pulmonary edema, tachycardia, hypotension/shock, syncope, torsade de pointes Endocrine/Metabolic: hyper- or hypoglycemia, especially in diabetic patients on insulin or oral hypoglycemic agents (see PRECAUTIONS, General and Drug Interactions). Gastrointestinal System: hepatic dysfunction including: hepatic necrosis, jaundice (cholestatic or hepatocellular), hepatitis; intestinal perforation; hepatic failure (including fatal cases); pseudomembranous colitis (the onset of pseudomembranous colitis symptoms may occur during or after antimicrobial treatment), GI hemorrhage; hiccough, painful oral mucosa, pyrosis (see WARNINGS). Genital/Reproductive System: vaginal candidiasis Hematopoietic: anemia, including hemolytic and aplastic; hemorrhage, pancytopenia, agranulocytosis, leukopenia, reversible bone marrow depression, thrombocytopenia, thrombotic thrombocytopenic purpura, petechiae, ecchymosis/bruising (see WARNINGS). Musculoskeletal: tendinitis/rupture; weakness; rhabdomyolysis (see WARNINGS). Nervous System: nightmares; suicidal thoughts or acts, disorientation, psychotic reactions, paranoia; phobia, agitation, restlessness, aggressiveness/hostility, manic reaction, emotional lability; peripheral neuropathy that may be irreversible, ataxia, incoordination; exacerbation of: myasthenia gravis and extrapyramidal disorders; dysphasia, lightheadedness (see WARNINGS and PRECAUTIONS). Respiratory System: dyspnea, bronchospasm, allergic pneumonitis, stridor (see WARNINGS). Skin/Hypersensitivity: anaphylactic (-toid) reactions/shock; purpura, serum sickness, erythema multiforme/Stevens-Johnson syndrome, erythema nodosum, exfoliative dermatitis, hyperpigmentation, toxic epidermal necrolysis, conjunctivitis, photosensitivity/phototoxicity reaction, vesiculobullous eruption (see WARNINGS and PRECAUTIONS). Special Senses: diplopia, nystagmus, blurred vision, disturbances of: taste, smell, hearing and equilibrium, usually reversible following discontinuation Urinary System: anuria, polyuria, renal calculi, renal failure, interstitial nephritis, hematuria (see WARNINGS and PRECAUTIONS). Laboratory
Hematopoietic: prolongation of prothrombin time Serum Chemistry: acidosis, elevation of: serum triglycerides, serum cholesterol, serum potassium, liver function tests including: GGTP, LDH, bilirubin Urinary: albuminuria, candiduria In clinical trials using multiple-dose therapy, ophthalmologic abnormalities, including cataracts and multiple punctate lenticular opacities, have been noted in patients undergoing treatment with other quinolones. The relationship of the drugs to these events is not presently established.
CRYSTALLURIA and CYLINDRURIA HAVE BEEN REPORTED with other quinolones.
-
OVERDOSAGEInformation on overdosage with ofloxacin is limited. One incident of accidental overdosage has been reported. In this case, an adult female received 3 grams of ofloxacin intravenously over 45 ...
Information on overdosage with ofloxacin is limited. One incident of accidental overdosage has been reported. In this case, an adult female received 3 grams of ofloxacin intravenously over 45 minutes. A blood sample obtained 15 minutes after the completion of the infusion revealed an ofloxacin level of 39.3 mcg/mL. In 7 h, the level had fallen to 16.2 mcg/mL, and by 24 h to 2.7 mcg/mL. During the infusion, the patient developed drowsiness, nausea, dizziness, hot and cold flushes, subjective facial swelling and numbness, slurring of speech, and mild to moderate disorientation. All complaints except the dizziness subsided within 1 h after discontinuation of the infusion. The dizziness, most bothersome while standing, resolved in approximately 9 h. Laboratory testing reportedly revealed no clinically significant changes in routine parameters in this patient.
In the event of an acute overdose, the stomach should be emptied. The patient should be observed and appropriate hydration maintained. Ofloxacin is not efficiently removed by hemodialysis or peritoneal dialysis.
Close -
DOSAGE AND ADMINISTRATIONThe usual dose of ofloxacin tablets is 200 mg to 400 mg orally every 12 h as described in the following dosing chart. These recommendations apply to patients with normal renal function (i.e. ...
The usual dose of ofloxacin tablets is 200 mg to 400 mg orally every 12 h as described in the following dosing chart. These recommendations apply to patients with normal renal function (i.e., creatinine clearance > 50 mL/min). For patients with altered renal function (i.e., creatinine clearance ≤ 50 mL/min), see the Patients With Impaired Renal Function subsection.
Infection* Unit Dose Frequency Duration Daily Dose - *
- DUE TO THE DESIGNATED PATHOGENS (see INDICATIONS AND USAGE).
Acute Bacterial Exacerbation of Chronic Bronchitis 400 mg q12h 10 days 800 mg Comm. Acquired Pneumonia 400 mg q12h 10 days 800 mg Uncomplicated Skin and Skin Structure Infections 400 mg q12h 10 days 800 mg Acute, Uncomplicated Urethral and Cervical Gonorrhea 400 mg single dose 1 day 400 mg Nongonococcal Cervicitis/Urethritis due to C. trachomatis 300 mg q12h 7 days 600 mg Mixed Infection of the Urethra and Cervix due to C. trachomatis and N. gonorrhoeae 300 mg q12h 7 days 600 mg Acute Pelvic Inflammatory Disease 400 mg q12h 10 to 14 days 800 mg Uncomplicated Cystitis due to E. coli or K. pneumoniae 200 mg q12h 3 days 400 mg Uncomplicated Cystitis due to Other Approved Pathogens 200 mg q12h 7 days 400 mg Complicated UTI's 200 mg q12h 10 days 400 mg Prostatitis due to E. coli 300 mg q12h 6 weeks 600 mg Antacids containing calcium, magnesium, or aluminum; sucralfate; divalent or trivalent cations such as iron; or multivitamins containing zinc; or didanosine, chewable/buffered tablets or the pediatric powder for oral solution should not be taken within the two-hour period before or within the two-hour period after taking ofloxacin (see PRECAUTIONS).
Patients With Impaired Renal Function
Dosage should be adjusted for patients with a creatinine clearance ≤ 50 mL/min. After a normal initial dose, dosage should be adjusted as follows:
Creatinine Clearance Maintenance Dose Frequency 20 to 50 mL/min the usual recommended unit dose q24h < 20 mL/min ½ the usual recommended unit dose q24h When only the serum creatinine is known, the following formula may be used to estimate creatinine clearance.
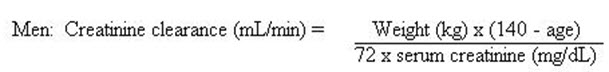
Women: 0.85 × the value calculated for men.
The serum creatinine should represent a steady-state of renal function.
ClosePatients With Cirrhosis
The excretion of ofloxacin may be reduced in patients with severe liver function disorders (e.g., cirrhosis with or without ascites). A maximum dose of 400 mg of ofloxacin per day should therefore not be exceeded.
-
HOW SUPPLIEDOfloxacin tablets USP, 200 mg are available as light yellow to yellow, oval, biconvex, film coated tablets, imprinted with "C213" in blue ink on one side and plain on the other side. They are ...
Ofloxacin tablets USP, 200 mg are available as light yellow to yellow, oval, biconvex, film coated tablets, imprinted with "C213" in blue ink on one side and plain on the other side. They are available in bottles of 50, 100 and 500 tablets.
Bottles of 50 (NDC 75834-199-50)
Bottles of 100 (NDC -75834-199-01)
Bottles of 500 (NDC 75834-199-05)Ofloxacin tablets USP, 300 mg are available as white to off white, oval, biconvex, film coated tablets, imprinted with "C212" in blue ink on one side and plain on the other side.. They are available in bottles of 50, 100 and 500 tablets.
Bottles of 50 (NDC 75834-200-50)
Bottles of 100 (NDC -75834-200-01)
Bottles of 500 (NDC -75834-200-05)Ofloxacin tablets USP, 400 mg are available as yellow to dark yellow, oval, biconvex, film coated tablets, imprinted with 'C211' in blue ink on one side and plain on other side. They are available in bottles of 50, 100 and 500 tablets.
Bottles of 50 (NDC -75834-201-50)
Bottles of 100 (NDC -75834-201-01)
Bottles of 500 (NDC -75834-201-05)CloseStore at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN.
-
ANIMAL PHARMACOLOGYOfloxacin, as well as other drugs of the quinolone class, has been shown to cause arthropathies (arthrosis) in immature dogs and rats. In addition, these drugs are associated with an increased ...
Ofloxacin, as well as other drugs of the quinolone class, has been shown to cause arthropathies (arthrosis) in immature dogs and rats. In addition, these drugs are associated with an increased incidence of osteochondrosis in rats as compared to the incidence observed in vehicle-treated rats (see WARNINGS). There is no evidence of arthropathies in fully mature dogs at intravenous doses up to 3 times the recommended maximum human dose (on a mg/m2 basis or 5 times based on mg/kg basis), for a one-week exposure period.
Long-term, high-dose systemic use of other quinolones in experimental animals has caused lenticular opacities; however, this finding was not observed in any animal studies with ofloxacin.
Reduced serum globulin and protein levels were observed in animals treated with other quinolones. In one ofloxacin study, minor decreases in serum globulin and protein levels were noted in female cynomolgus monkeys dosed orally with 40 mg/kg ofloxacin daily for one year. These changes, however, were considered to be within normal limits for monkeys.
Crystalluria and ocular toxicity were not observed in any animals treated with ofloxacin.
Close -
REFERENCESClinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard - Ninth Edition. CLSI document ...
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard - Ninth Edition. CLSI document M07-A9, Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2012.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Diffusion Susceptibility Tests; Approved Standards – Eleventh Edition. CLSI document M02-A11, Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2012.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-third Informational Supplement, CLSI document M100-S24, Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2014.
-
SPL UNCLASSIFIED SECTIONManufactured By: Cadila Pharmaceuticals Limited - 1389, Dholka, District - Ahmedabad, Gujarat State, INDIA - Manufactured for: Nivagen Pharmaceuticals, Inc. Sacramento, CA 95827 - Toll free number ...
Manufactured By:
Cadila Pharmaceuticals Limited
1389, Dholka, District - Ahmedabad,
Gujarat State, INDIAManufactured for:
Nivagen Pharmaceuticals, Inc.
Sacramento, CA 95827
Toll free number: 1-877-977-0687Revised: December 2018
Close -
MEDICATION GUIDEOfloxacin Tablets, USP - (oh-FLOX-a-sin) Rx only - Read the Medication Guide that comes with ofloxacin before you start taking it and each time you get a refill. There may be new information. This ...
Ofloxacin Tablets, USP
(oh-FLOX-a-sin)Rx only
Read the Medication Guide that comes with ofloxacin before you start taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about ofloxacin tablets?
Ofloxacin belongs to a class of antibiotics called fluoroquinolones. Ofloxacin tablets can cause serious side effects. Some of these serious side effects can happen at the same time and could result in death. If you get any of the following serious side effects, get medical help right away. Talk with your healthcare provider about whether you should continue to take ofloxacin tablets.
1. Tendon rupture or swelling of the tendon (tendinitis).
-
Tendon problems can happen in people of all ages who take ofloxacin tablets. Tendons are tough cords of tissue that connect muscles to bones.
- Pain, swelling, tears, and inflammation of tendons including the back of the ankle (Achilles), shoulder, hand, or other tendon sites.
-
The risk of getting tendon problems while you take ofloxacin tablets is higher if you:
- are over 60 years of age
- are taking steroids (corticosteroids)
- have had a kidney, heart or lung transplant.
-
Tendon problems can happen in people who do not have the above risk factors when they take ofloxacin tablets. Other reasons that can increase your risk of tendon problems can include:
- physical activity or exercise
- kidney failure
- tendon problems in the past, such as in people with rheumatoid arthritis (RA).
- Stop taking ofloxacin immediately and get medical help right away at the first sign of tendon pain, swelling or inflammation. Stop taking ofloxacin tablets until tendinitis or tendon rupture has been ruled out by your healthcare provider. Avoid exercise and using the affected area. The most common area of pain and swelling is the Achilles tendon at the back of your ankle. This can also happen with other tendons.
- Talk to your healthcare provider about the risk of tendon rupture with continued use of ofloxacin tablets. You may need a different antibiotic that is not a fluoroquinolone to treat your infection.
- Tendon rupture can happen while you are taking or after you have finished taking ofloxacin tablets. Tendon ruptures have happened up to several months after patients have finished taking their fluoroquinolone.
-
Get medical help right away if you get any of the following signs or symptoms of a tendon rupture:
- hear or feel a snap or pop in a tendon area
- bruising right after an injury in a tendon area
- unable to move the affected area or bear weight
2. Changes in sensation and possible nerve damage (Peripheral Neuropathy). Damage to the nerves in arms, hands, legs, or feet can happen in people who take fluoroquinolones, including ofloxacin. Stop taking ofloxacin immediately and talk to your healthcare provider right away if you get any of the following symptoms of peripheral neuropathy in your arms, hands, legs, or feet:
- pain
- burning
- tingling
- numbness
- weakness
The nerve damage may be permanent.
3. Central Nervous System (CNS) effects. Seizures have been reported in people who take fluoroquinolone antibacterial medicines, including ofloxacin. Tell your healthcare provider if you have a history of seizures before you start taking ofloxacin. CNS side effects may happen as soon as after taking the first dose of ofloxacin. Stop taking ofloxacin immediately and talk to your healthcare provider right away if you get any of these side effects, or other changes in mood or behavior:
- seizures
- hear voices, see things, or sense things that are not there (hallucinations)
- feel restless
- tremors
- feel anxious or nervous
- confusion
- depression
- trouble sleeping
- nightmares
- feel lightheaded or dizzy
- feel more suspicious (paranoia)
- suicidal thoughts or acts
- headaches that will not go away, with or without blurred vision
4. Worsening of myasthenia gravis (a disease that causes muscle weakness). Fluoroquinolones like ofloxacin tablets may cause worsening of myasthenia gravis symptoms, including muscle weakness and breathing problems. Call your healthcare provider right away if you have any worsening muscle weakness or breathing problems.
See the section "What are the possible side effects of ofloxacin tablets?" for more information about side effects.
What is ofloxacin?
Ofloxacin tablets are a fluoroquinolone antibiotic medicine used in adults to treat certain infections caused by certain germs called bacteria. It is not known if ofloxacin tablets are safe and work in people under 18 years of age. Children less than 18 years of age have a higher chance of getting bone, joint, or tendon (musculoskeletal) problems such as pain or swelling while taking ofloxacin tablets.
Sometimes infections are caused by viruses rather than by bacteria. Examples include viral infections in the sinuses and lungs, such as the common cold or flu. Antibiotics including ofloxacin tablets do not kill viruses.
Call your healthcare provider if you think your condition is not getting better while you are taking ofloxacin tablets.
Who should not take ofloxacin tablets?
Do not take ofloxacin tablets if you have ever had a severe allergic reaction to an antibiotic known as a fluoroquinolone, or if you are allergic to any of the ingredients in ofloxacin. Ask your healthcare provider if you are not sure. See the list of the ingredients in ofloxacin tablets at the end of this Medication Guide.
What should I tell my healthcare provider before taking ofloxacin tablets?
See "What is the most important information I should know about ofloxacin tablets?"
Tell your healthcare provider about all your medical conditions, including if you:
- have tendon problems-ofloxacin should not be used in patients who have a history of tendon problems
- have a disease that causes muscle weakness (myasthenia gravis)
- have central nervous system problems (such as epilepsy)
- have nerve problems-ofloxacin should not be used in patients who have a history of nerve problems called peripheral neuropathy
- have or anyone in your family has an irregular heartbeat, especially a condition called "QT prolongation."
- have low blood potassium (hypokalemia)
- have a history of seizures
- have kidney problems. You may need a lower dose of ofloxacin tablets if your kidneys do not work well.
- have liver problems
- have rheumatoid arthritis (RA) or other history of joint problems
- are pregnant or planning to become pregnant. It is not known if ofloxacin tablets will harm your unborn child
- are breastfeeding or planning to breastfeed. Ofloxacin passes into breast milk. You and your healthcare provider should decide whether you will take ofloxacin tablets or breastfeed.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, herbal and dietary supplements. Ofloxacin tablets and other medicines can affect each other causing side effects. Especially tell your healthcare provider if you take:
- an NSAID (Non-Steroidal Anti-Inflammatory Drug). Many common medicines for pain relief are NSAIDs. Taking an NSAID while you take ofloxacin tablets or other fluoroquinolones may increase your risk of central nervous system effects and seizures. See "What are the possible side effects of ofloxacin tablets?"
- theophylline
- a blood thinner (warfarin, Coumadin®, Jantoven®)
- an oral anti-diabetes medicine or insulin
- a medicine to control your heart rate or rhythm (antiarrhythmics). See "What are the possible side effects of ofloxacin tablets".
- an anti-psychotic medicine
- a tricyclic antidepressant
- a water pill (diuretic)
- a steroid medicine. Corticosteroids taken by mouth or by injection may increase the chance of tendon injury. See "What is the most important information I should know about ofloxacin tablets?".
- Certain medicines may keep ofloxacin tablets from working correctly. Take ofloxacin tablets either 2 hours before or 2 hours after taking these products:
- an antacid, multivitamin, or other product that has magnesium, aluminum, iron, or zinc.
- sucralfate (Carafate®)
- didanosine (Videx®, Videx® EC)
Ask your healthcare provider if you are not sure if any of your medicines are listed above. Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take ofloxacin tablets?
- Take ofloxacin tablets exactly as prescribed by your healthcare provider.
- Take ofloxacin tablets at about the same time each day.
- Drink plenty of fluids while taking ofloxacin tablets.
- Ofloxacin tablets can be taken with or without food.
- Do not skip any doses, or stop taking ofloxacin tablets , even if you begin to feel better, until you finish your prescribed treatment, unless:
- you have tendon effects (see "What is the most important information I should know about ofloxacin tablets ?"),
- you have nerve problems (see "What is the most important information I should know about ofloxacin tablets?")
- you have central nervous system problems (see "What is the most important information I should know about ofloxacin tablets?")
- you have a serious allergic reaction (see "What are the possible side effects of ofloxacin tablets ?"), or
- your healthcare provider tells you to stop.
- This will help make sure that all of the bacteria are killed and lower the chance that the bacteria will become resistant to ofloxacin tablets. If this happens, ofloxacin tablets and other antibiotic medicines may not work in the future.
- If you miss a dose of ofloxacin tablets, take it as soon as you remember. Do not take two doses of ofloxacin tablets at the same time. Do not take more than two doses in one day.
- If you take too much, call your healthcare provider or get medical help immediately.
What should I avoid while taking ofloxacin tablets?
- Ofloxacin tablets can make you feel dizzy and lightheaded. Do not drive, operate machinery, or do other activities that require mental alertness or coordination until you know how ofloxacin tablets affect you.
- Avoid sunlamps, tanning beds, and try to limit your time in the sun. Ofloxacin tablets can make your skin sensitive to the sun (photosensitivity) and the light from sunlamps and tanning beds. You could get severe sunburn, blisters or swelling of your skin. If you get any of these symptoms while taking ofloxacin tablets, call your healthcare provider right away. You should use a sunscreen and wear a hat and clothes that cover your skin if you have to be in sunlight.
What are the possible side effects of ofloxacin tablets?
Ofloxacin tablets can cause side effects that may be serious or even cause death. See "What is the most important information I should know about ofloxacin tablets?"
Other serious side effects of ofloxacin tablets include:
-
Serious allergic reactions.
Allergic reactions can happen in people taking fluoroquinolones, including ofloxacin tablets, even after only one dose. Stop taking ofloxacin tablets and get emergency medical help right away if you get any of the following symptoms of a severe allergic reaction:- hives
- trouble breathing or swallowing
- swelling of the lips, tongue, face
- throat tightness, hoarseness
- rapid heartbeat
- faint
- yellowing of the skin or eyes. Stop taking ofloxacin tablets and tell your healthcare provider right away if you get yellowing of your skin or white part of your eyes, or if you have dark urine. These can be signs of a serious reaction to ofloxacin tablets (a liver problem).
- Aortic aneurysm and dissection
People who take fluoroquinolone medicines, including ofloxacin tablets, have an increased risk of swelling of the large artery that carries blood from the heart to the body (aortic aneurysm) and tearing (dissection) of this artery. Tell your healthcare provider if you have ever been told that you have an aortic aneurysm. Get emergency medical help right away if you have sudden chest, stomach, or back pain. -
Skin rash
Skin rash may happen in people taking ofloxacin tablets, even after only one dose. Stop taking ofloxacin tablets at the first sign of a skin rash and call your healthcare provider. Skin rash may be a sign of a more serious reaction to ofloxacin. -
Intestine infection (Pseudomembranous colitis)
Pseudomembranous colitis can happen with most antibiotics, including ofloxacin tablets. Call your healthcare provider right away if you get watery diarrhea, diarrhea that does not go away, or bloody stools. You may have stomach cramps and a fever. Pseudomembranous colitis can happen 2 or more months after you have finished your antibiotic. -
Serious heart rhythm changes (QT prolongation and torsade de pointes)
Tell your healthcare provider right away if you have a change in your heart beat (a fast or irregular heartbeat), or if you faint. Ofloxacin tablets may cause a rare heart problem known as prolongation of the QT interval. This condition can cause an abnormal heartbeat and can be very dangerous. The chances of this happening are higher in people:- who are elderly
- with a family history of prolonged QT interval
- with low blood potassium (hypokalemia)
- who take certain medicines to control heart rhythm (antiarrhythmics)
- Sensitivity to sunlight (photosensitivity): See "What should I avoid while taking ofloxacin tablets?"
- Low blood sugar (hypoglycemia). People who take ofloxacin tablets and other fluoroquinolone medicines with oral anti-diabetes medicines or with insulin can get low blood sugar (hypoglycemia). Follow your healthcare provider's instructions for how often to check your blood sugar. If you have diabetes and you get low blood sugar while taking ofloxacin tablets, stop taking ofloxacin tablets right away and call your healthcare provider right away. Your antibiotic medicine may need to be changed.
-
Changes in blood sugar
People who take fluoroquinolone medicines with oral anti-diabetes medicines or with insulin can get low blood sugar (hypoglycemia) and high blood sugar (hyperglycemia). Follow your healthcare provider's instructions for how often to check your blood sugar. If you have diabetes and you get low blood sugar while taking ofloxacin, stop taking ofloxacin and call your healthcare provider right away. Your antibiotic medicine may need to be changed.
The most common side effects of ofloxacin tablets include:
- Sleep problems
- headache
- dizziness
- nausea
- vomiting
- diarrhea
- itching
- external genital itching in women
- vaginal inflammation (vaginitis)
- taste changes
Ofloxacin tablets may cause false-positive urine screening results for opiates when testing is done with some commercially available kits. A positive result should be confirmed using a more specific test.
These are not all the possible side effects of ofloxacin tablets. Tell your healthcare provider about any side effect that bothers you or that does not go away.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ofloxacin tablets?
- Store ofloxacin tablets at 20° to 25° C (68°F to 77°F).
- Keep the bottle that ofloxacin tablets come in closed tightly.
- Keep ofloxacin tablets and all medicines out of the reach of children.
General Information about ofloxacin tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ofloxacin tablets for a condition for which it is not prescribed. Do not give ofloxacin tablets to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about ofloxacin tablets. If you would like more information about ofloxacin tablets, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about ofloxacin tablets that is written for healthcare professionals. For more information go to www.cadilapharma.com or call 1-202-355-9785.
What are the ingredients in ofloxacin tablets?
- Active ingredient: ofloxacin, USP
- Inactive ingredients: lactose monohydrate, pregelatinized maize starch, hydroxy propyl methyl cellulose, talc, magnesium stearate, polyethylene glycol, sodium starch glycolate, and titanium dioxide. Additionally, the 200 mg and 400 mg tablets contain iron oxide yellow. The imprinting ink for 200 mg, 300 mg and 400 mg strength contains FD&C blue #1, isopropyl alcohol, N-butyl alcohol, propylene glycol, shellac and titanium dioxide.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
All brand names listed are the registered trademarks of their respective owners and are not trademarks of Cadila Pharmaceuticals Limited, India.
Manufactured by:
Cadila Pharmaceuticals Limited
1389, Dholka, District - Ahmedabad,
Gujarat State, INDIAManufactured for:
Nivagen Pharmaceuticals, Inc.
Sacramento, CA 95827
Toll free number: 1-877-977-0687Revised: December 2018
Close -
Tendon problems can happen in people of all ages who take ofloxacin tablets. Tendons are tough cords of tissue that connect muscles to bones.
-
PRINCIPAL DISPLAY PANEL - 200 mg Tablet Bottle LabelNIVAGEN - PHARMACEUTICALS - NDC 75834-199-50 - Ofloxacin - Tablets USP - 200 mg - 50 Tablets - Rx Only
-
PRINCIPAL DISPLAY PANEL - 300 mg Tablet Bottle LabelNIVAGEN - PHARMACEUTICALS - NDC 75834-200-50 - Ofloxacin - Tablets USP - 300 mg - 50 Tablets - Rx Only
-
PRINCIPAL DISPLAY PANEL - 400 mg Tablet Bottle LabelNIVAGEN - PHARMACEUTICALS - NDC 75834-201-50 - Ofloxacin - Tablets USP - 400 mg - 50 Tablets - Rx Only
-
INGREDIENTS AND APPEARANCEProduct Information
OFLOXACIN ofloxacin tablet, coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:75834-199 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OFLOXACIN (UNII: A4P49JAZ9H) (OFLOXACIN - UNII:A4P49JAZ9H) OFLOXACIN 200 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) TALC (UNII: 7SEV7J4R1U) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color YELLOW (LIGHT YELLOW) Score no score Shape OVAL Size 15mm Flavor Imprint Code C213 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75834-199-50 50 in 1 BOTTLE; Type 0: Not a Combination Product 01/25/2019 2 NDC:75834-199-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/25/2019 3 NDC:75834-199-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 01/25/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091656 01/25/2019 OFLOXACIN ofloxacin tablet, coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:75834-200 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OFLOXACIN (UNII: A4P49JAZ9H) (OFLOXACIN - UNII:A4P49JAZ9H) OFLOXACIN 300 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) TALC (UNII: 7SEV7J4R1U) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (OFF WHITE) Score no score Shape OVAL Size 17mm Flavor Imprint Code C212 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75834-200-50 50 in 1 BOTTLE; Type 0: Not a Combination Product 01/25/2019 2 NDC:75834-200-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/25/2019 3 NDC:75834-200-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 01/25/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091656 01/25/2019 OFLOXACIN ofloxacin tablet, coated Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:75834-201 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OFLOXACIN (UNII: A4P49JAZ9H) (OFLOXACIN - UNII:A4P49JAZ9H) OFLOXACIN 400 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) TALC (UNII: 7SEV7J4R1U) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color YELLOW (DARK YELLOW) Score no score Shape OVAL Size 19mm Flavor Imprint Code C211 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75834-201-50 50 in 1 BOTTLE; Type 0: Not a Combination Product 01/25/2019 2 NDC:75834-201-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 01/25/2019 3 NDC:75834-201-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 01/25/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091656 01/25/2019
CloseLabeler - Nivagen Pharmaceuticals, Inc. (052032418)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
OFLOXACIN tablet, coated
Number of versions: 2
| Published Date (What is this?) | Version | Files |
|---|---|---|
| Jul 27, 2023 | 2 (current) | download |
| Feb 6, 2019 | 1 | download |
RxNorm
OFLOXACIN tablet, coated
Get Label RSS Feed for this Drug
OFLOXACIN tablet, coated
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=1779c568-d7bb-4bd5-bc29-13bd52ba8a0a
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
OFLOXACIN tablet, coated
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 75834-199-01 |
| 2 | 75834-199-05 |
| 3 | 75834-199-50 |
| 4 | 75834-200-01 |
| 5 | 75834-200-05 |
| 6 | 75834-200-50 |
| 7 | 75834-201-01 |
| 8 | 75834-201-05 |
| 9 | 75834-201-50 |