Label: VERAPAMIL HYDROCHLORIDE tablet
- NDC Code(s): 75834-158-01, 75834-158-05, 75834-159-01, 75834-159-05, view more
- Packager: Nivagen Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 18, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
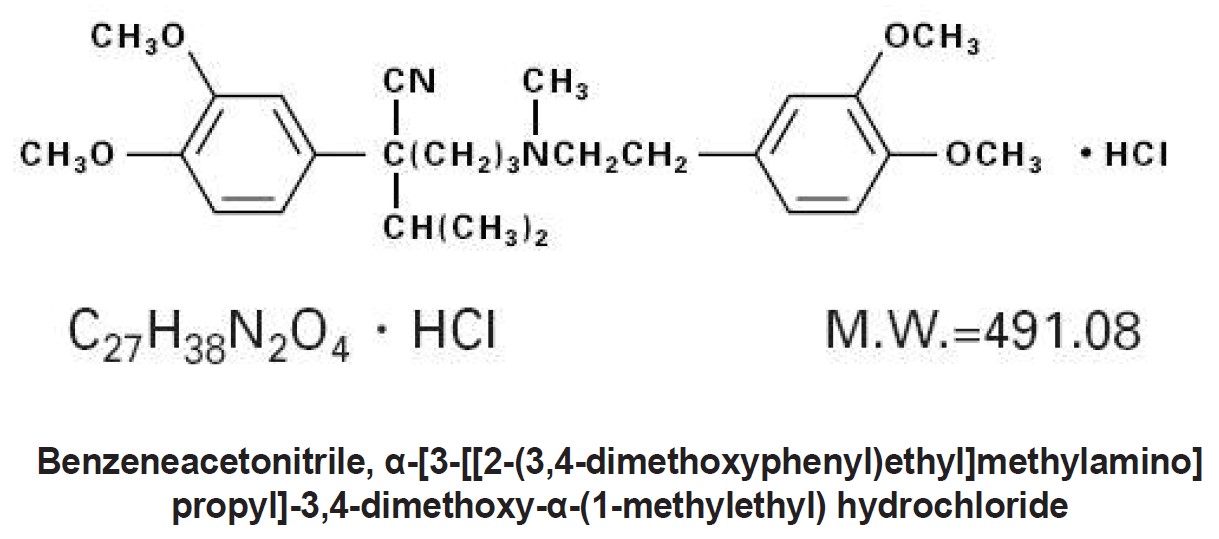
DESCRIPTIONVerapamil hydrochloride extended-release tablets, USP is a calcium ion influx inhibitor (slow-channel blocker or calcium ion antagonist). Verapamil hydrochloride extended-release tablets, USP is ...
-
CLINICAL PHARMACOLOGYVerapamil HCl is a calcium ion influx inhibitor (slow-channel blocker or calcium ion antagonist) that exerts its pharmacologic effects by modulating the influx of ionic calcium across the cell ...
-
INDICATIONS AND USAGEVerapamil hydrochloride extended-release tablets is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal ...
-
CONTRAINDICATIONSVerapamil HCl extended-release tablets are contraindicated in: 1.Severe left ventricular dysfunction (see WARNINGS) 2.Hypotension (systolic pressure less than 90 mm Hg) or cardiogenic shock ...
-
WARNINGSHeart failure: Verapamil has a negative inotropic effect, which in most patients is compensated by its afterload reduction (decreased systemic vascular resistance) properties without a net ...
-
PRECAUTIONSGeneral - Use in patients with impaired hepatic function: Since verapamil is highly metabolized by the liver, it should be administered cautiously to patients with impaired hepatic function ...
-
ADVERSE REACTIONSSerious adverse reactions are uncommon when verapamil therapy is initiated with upward dose titration within the recommended single and total daily dose. See WARNINGS for discussion of heart ...
-
OVERDOSAGEOverdose with verapamil may lead to pronounced hypotension, bradycardia, and conduction system abnormalities (eg, junctional rhythm with AV dissociation and high degree AV block, including ...
-
DOSAGE & ADMINISTRATIONEssential hypertension: The dose of verapamil hydrochloride extended-release tablets should be individualized by titration and the drug should be administered with food. Initiate therapy with 180 ...
-
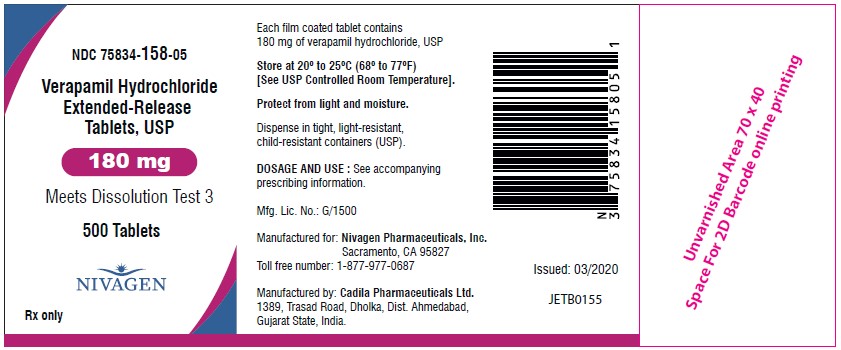
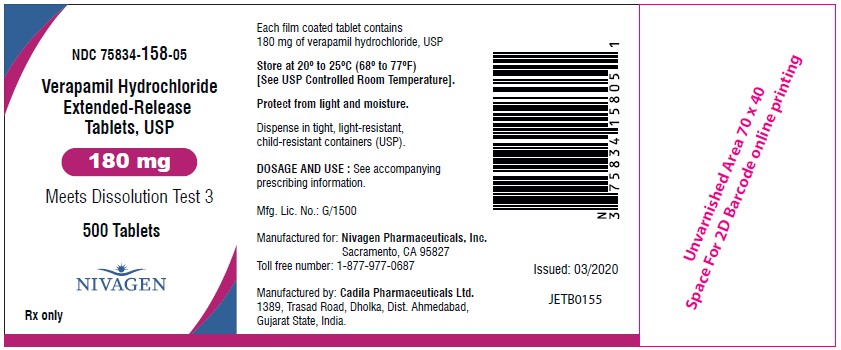
HOW SUPPLIEDVerapamil hydrochloride extended-release tablets, USP 120 mg are supplied as light blue, oval shaped, film coated tablets debossed with "V12" on one side and plain on other side. NDC ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 75834-320-01 - Verapamil Hydrochloride Extended-Release Tablets, USP (120 mg) Meets Dissolution Test 3 - 100 Tablets - Nivagen Pharmaceuticals Inc. Rx only - NDC 75834-320-05 - Verapamil ...
-
INGREDIENTS AND APPEARANCEProduct Information