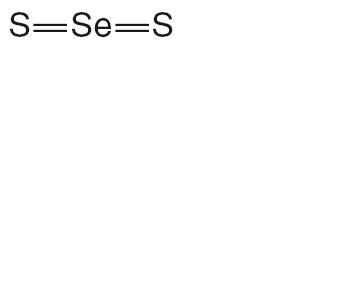Label: TERSI- selenium sulfide aerosol, foam
-
Contains inactivated NDC Code(s)
NDC Code(s): 23710-225-01, 23710-225-70 - Packager: Quinnova Pharmaceuticals, Inc.
- Category: NON-STANDARDIZED ALLERGENIC LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
Drug Label Information
Updated December 4, 2009
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
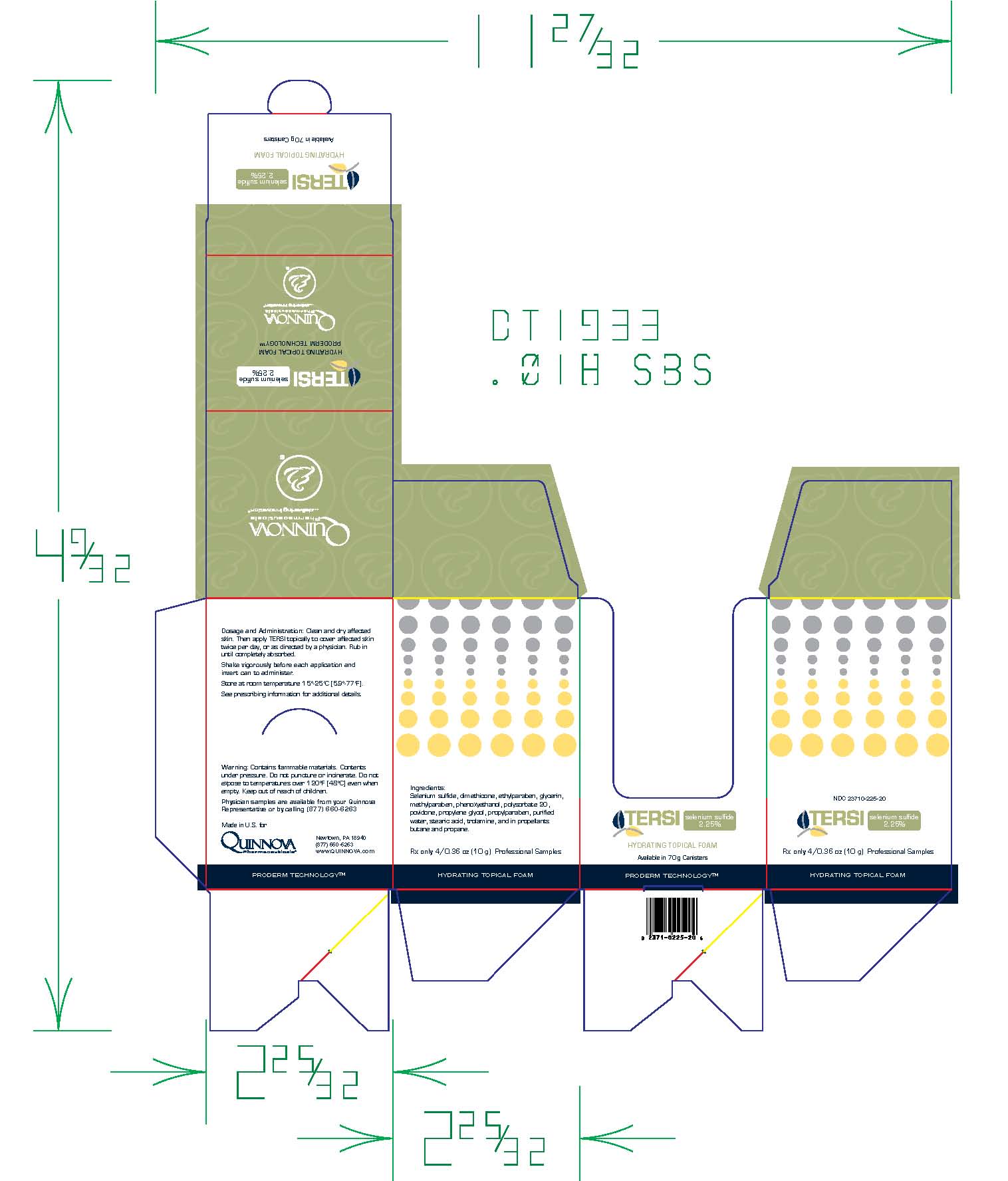
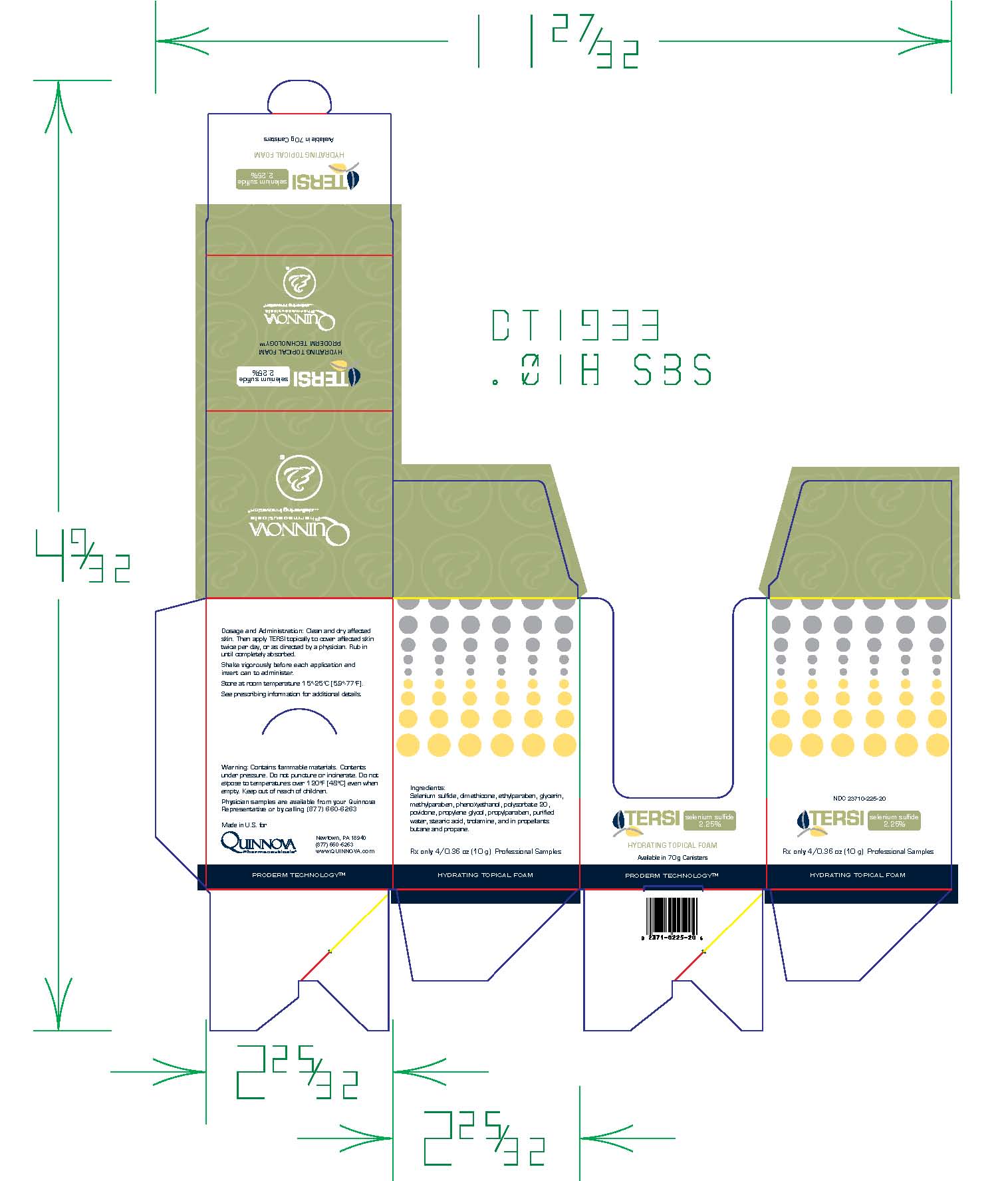
TERSI, which is applied topically, is an antiseborrheic, antifungal preparation for the treatment of seborrheic dermatitis and tinea versicolor of the skin. Each gram of TERSI contains selenium sulfide 2.25% as the active ingredient, and the following inactive ingredients: dimethicone, ethylparaben, glycerin, methylparaben, phenoxyethanol, polysorbate 20, povidone, propylene glycol, propylparaben, purified water, stearic acid, trolamine, and in propellants butane and propane.
- CLINICAL PHARMACOLOGY
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
TERSI is for external use only. It is not for ophthalmic, oral, anal or intravaginal use. Contact with eyes, lips, broken or inflamed skin, and all mucous membranes should be avoided. TERSI should not be used by persons who have a known hypersensitivity to selenium sulfide or any of the other listed ingredients.
-
PRECAUTIONS
TERSI should be used only as directed by a physician and should not be used to treat any condition other than that for which it is prescribed.TERSI should not be used on any skin area where inflammation or exudation is present as increased absorption may occur. If redness or irritation occurs, discontinue use and consult with prescribing physician.
Pregnancy (Category C)
Animal reproduction studies have not been performed with topically applied selenium sulfide and it is not known whether TERSI can cause fetal harm when administered to a pregnant woman. Nevertheless, TERSI should be used by a pregnant woman only if necessary.Nursing Mothers
It is not known whether topically applied selenium sulfide is excreted in human milk. Due to the fact that many drugs are excreted in human milk, caution should be exercised by physicians when administering TERSI to nursing mothers.
KEEP THIS AND ALL OTHER MEDICATIONS OUT OF THE REACH OF CHILDREN. - ADVERSE REACTIONS
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
TERSI is supplied in a 70 gram or 2.5 ounce aerosolized canister bearing the NDC Number 23710-225-70 and a 10 gram or 0.36 ounce aerosolized canister bearing the NDC Number 23710-025-01. The 10 gram canister is physician dispensed sample product.
Store at controlled room temperature 15º - 25ºC (59º - 77ºF).
Contains flammable materials. Contents under pressure. Do not puncture or incinerate. Do not expose to temperatures over 120ºF [48ºC] even when empty. Keep out of reach of children.
- SPL UNCLASSIFIED SECTION
- Can Label
- Folding Carton
-
INGREDIENTS AND APPEARANCE
TERSI
selenium sulfide aerosol, foamProduct Information Product Type NON-STANDARDIZED ALLERGENIC Item Code (Source) NDC:23710-225 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SELENIUM SULFIDE (UNII: Z69D9E381Q) (SELENIUM - UNII:H6241UJ22B) SELENIUM SULFIDE 1.44 g in 70 g Inactive Ingredients Ingredient Name Strength POVIDONE K29/32 (UNII: 390RMW2PEQ) STEARIC ACID (UNII: 4ELV7Z65AP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERIN (UNII: PDC6A3C0OX) DIMETHICONE (UNII: 92RU3N3Y1O) POLYSORBATE 20 (UNII: 7T1F30V5YH) ETHYLPARABEN (UNII: 14255EXE39) PROPYLPARABEN (UNII: Z8IX2SC1OH) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) WATER (UNII: 059QF0KO0R) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:23710-225-70 70 g in 1 CANISTER 2 NDC:23710-225-01 10 g in 1 CANISTER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 12/01/2009 Labeler - Quinnova Pharmaceuticals, Inc. (607183766)