Label: CAPSINAC- diclofenac sodium and capsaicin kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 51021-250-05, 51021-302-08, 73247-201-01 - Packager: Bodysphere, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 26, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Diclofenac Sodium Topical Solution safely and effectively. See full prescribing information for Diclofenac Sodium Topical Solution ...
-
Table of ContentsTable of Contents
-
BOXED WARNING
(What is this?)Cardiovascular Risk - Nonsteroidal anti-inflammatory drugs (NSAIDs) may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal ...
WARNING: CARDIOVASCULAR AND GASTROINTESTINAL RISK
Cardiovascular Risk
- Nonsteroidal anti-inflammatory drugs (NSAIDs) may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk [see Warnings and Precautions (5.1)].
- Diclofenac sodium topical solution is contraindicated in the perioperative setting of coronary artery bypass graft (CABG) surgery [see Contraindications (4)].
CloseGastrointestinal Risk
- NSAIDs cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events [see Warnings and Precautions (5.2)].
-
1. INDICATIONS AND USAGEDiclofenac sodium topical solution is a nonsteroidal anti-inflammatory drug (NSAID) indicated for the treatment of signs and symptoms of osteoarthritis of the knee(s).
-
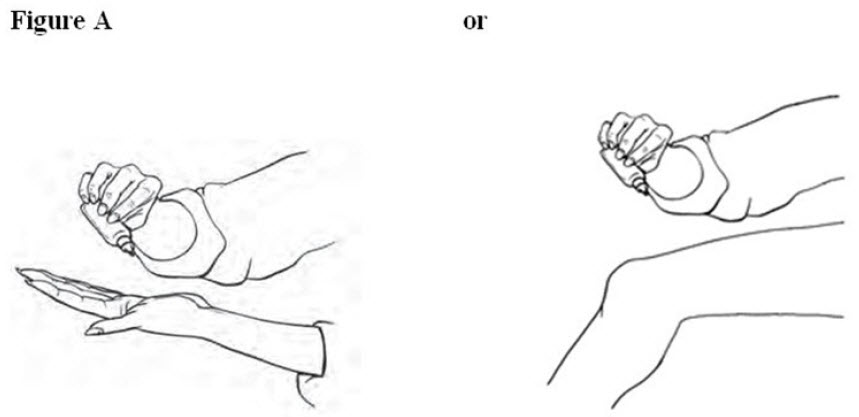
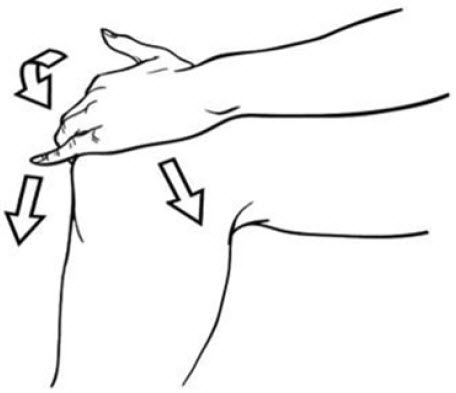
2. DOSAGE AND ADMINISTRATION2.1 General Instructions - For the relief of the signs and symptoms of osteoarthritis of the knee(s), the recommended dose is 40 drops per knee, 4 times a day. Apply diclofenac sodium topical ...
-
3. DOSAGE FORMS AND STRENGTHS1.5% w/w topical solution
-
4. CONTRAINDICATIONSDiclofenac sodium topical solution is contraindicated in patients with a known hypersensitivity to diclofenac sodium or any other component of diclofenac sodium topical solution. Diclofenac sodium ...
-
5. WARNINGS AND PRECAUTIONS5.1 Cardiovascular Thrombotic Events - Clinical trials of several oral COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular ...
-
6. ADVERSE REACTIONS6.1 Clinical Studies Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of another drug cannot be directly ...
-
7. DRUG INTERACTIONSDrug interactions with the use of diclofenac sodium topical solution have not been studied. The following drug interactions [sections 7.1 to 7.7] are noted for oral diclofenac sodium. 7.1 ...
-
8. USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Category C prior to 30 weeks gestation; Category D starting 30 weeks gestation. Teratogenic Effects: There are no adequate and well-controlled studies of diclofenac ...
-
10. OVERDOSAGEThere have been no known experiences of overdose with diclofenac sodium topical solution. Symptoms following acute NSAID overdose are usually limited to lethargy, drowsiness, nausea, vomiting, and ...
-
11. DESCRIPTIONDiclofenac sodium topical solution is a clear, colorless to faintly pink-orange solution for topical application. Diclofenac sodium topical solution contains 1.5% w/w diclofenac sodium, a ...
-
12. CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of diclofenac is similar to that of other nonsteroidal anti-inflammatory drugs. Diclofenac inhibits the enzyme, cyclooxygenase (COX), an early ...
-
13. NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenicity studies in mice and rats administered diclofenac sodium, as a dietary constituent for 2 years resulted in no ...
-
14. CLINICAL STUDIES14.1 Pivotal Studies in Osteoarthritis of the Knee - The use of diclofenac sodium topical solution for the treatment of the signs and symptoms of osteoarthritis of the knee was evaluated in two ...
-
16. HOW SUPPLIED/STORAGE AND HANDLINGDiclofenac Sodium Topical Solution is supplied as a clear, colorless to faintly pink-orange solution containing 16.05 mg of diclofenac sodium per mL of solution, in a white high density ...
-
17. PATIENT COUNSELING INFORMATIONSee FDA-Approved Patient Labeling (Medication Guide and Instructions for Use). 17.1 Patient/Caregiver Instructions - Inform patients of the following information before initiating therapy with ...
-
Medication Guide for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)(See the end of this Medication Guide for a list of prescription NSAID medicines.) What is the most important information I should know about medicines called Non-Steroidal Anti-Inflammatory ...
-
INSTRUCTIONS FOR USE17.11 Patient Instructions for Use - Diclofenac Sodium Topical Solution - Read the Medication Guide that comes with diclofenac sodium topical solution first. Be sure that you read, understand, and ...
-
SPL UNCLASSIFIED SECTIONCapsaicin Cream - (Capsaicin 0.025%)
-
Active ingredientCapsicum Oleoresin 0.0625% (containing capsaicin 0.025%)
-
PurposeTopical Analgesic
-
UsesFor the temporary relief of minor aches and pains of muscles and joints associated with arthritis, strains, and sprains.
-
WarningsFor external use only - This is not a face cream. Do not apply to the face. Do not use if allergic to chili peppers or if past allergic reaction to capsaicin. Do not apply to wounds or damaged ...
-
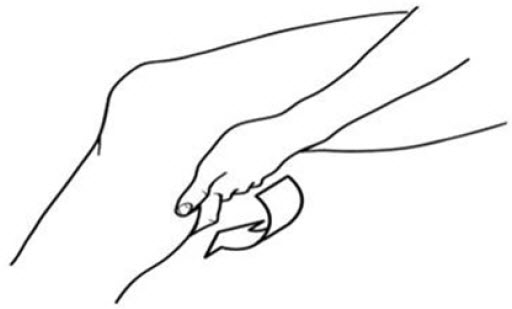
DirectionsRotate pump's spout counter-clockwise slightly to unlock; clockwise to lock. Before using on children under 18 years of age, consult a physician. Apply sparingly to affected area not more than 4 ...
-
Other informationWARNING: FLAMMABLE PRODUCT - Store in a cool well ventilated area away from heat. Keep away from sparks or open flame.
-
Inactive ingredientsAloe Barbadensis Leaf Extract, Bisabolol, Carbomer, Caprylyl Glycol, Cetearyl Alcohol, Ceteareth-20, Green Tea (Camellia Sinensis) Leaf Extract, Glycerin, Methylisothiazolinone, PEG-8 ...
-
Questions or comments ?Call toll-free1-888-452-9975 or visit us at www.sirclelabs.com - Manufactured for: Sircle Laboratories, LLC, Madison, MS 39110
-
CAPSINAC Therapy Kit (NDC 73247-201-01)How Supplied: 1 unit (pack); NDC 73247-201-01 - Each kit contains: 1 - Diclofenac Sodium 1.5% Topical Solution (150ml bottle) 1 - Capsaicin 0.025% Cream (237ml bottle) *See individual products ...
-
PRINCIPAL DISPLAY PANEL - Kit CartonRx Only - NDC 73247-201-01 - CAPSINAC - Therapy Kit - Diclofenac Sodium Topical Solution - (1.5% w/w, 5 fl oz/150mL) Capsaicin Cream - (Capsaicin 0.025%, 8 fl oz/237mL) TOPICAL ONLY - BODYSPHERE + MADE IN ...
-
INGREDIENTS AND APPEARANCEProduct Information