Label: DIHYDROERGOTAMINE MESYLATE spray
- NDC Code(s): 72888-096-18, 72888-096-19, 72888-096-34
- Packager: Advagen Pharma Ltd
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx Only
-
BOXED WARNING
(What is this?)
WARNING: PERIPHERAL ISCHEMIA FOLLOWING COADMINISTRATION
WITH POTENT CYP3A4 INHIBITORSSerious and/or life-threatening peripheral ischemia has been associated with the coadministration of DIHYDROERGOTAMINE with potent CYP 3A4 inhibitors including protease inhibitors and macrolide antibiotics. Because CYP 3A4 inhibition elevates the serum levels of DIHYDROERGOTAMINE, the risk for vasospasm leading to cerebral ischemia and/or ischemia of the extremities is increased. Hence, concomitant use of these medications is contraindicated.
(See also CONTRAINDICATIONSand WARNINGSsection)
Close -
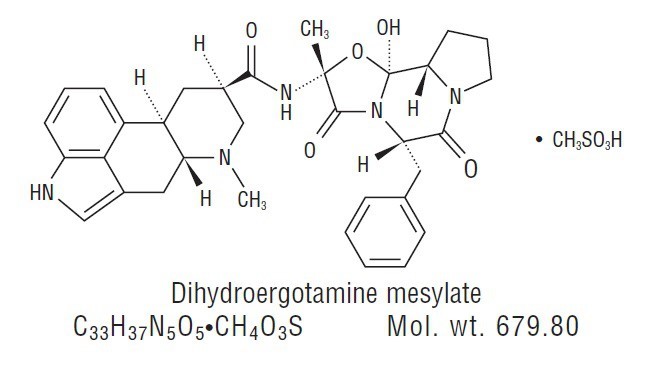
DESCRIPTIONDihydroergotamine mesylate is ergotamine hydrogenated in the 9,10 position as the mesylate salt. Dihydroergotamine mesylate is known chemically as ergotaman-3', 6', 18-trione ...
-
CLINICAL PHARMACOLOGYMechanism of Action - Dihydroergotamine binds with high affinity to 5-HT - 1Dαand 5-HT - 1Dβreceptors. It also binds with high affinity to serotonin 5-HT - 1A,5-HT - 2A,and 5-HT ...
-
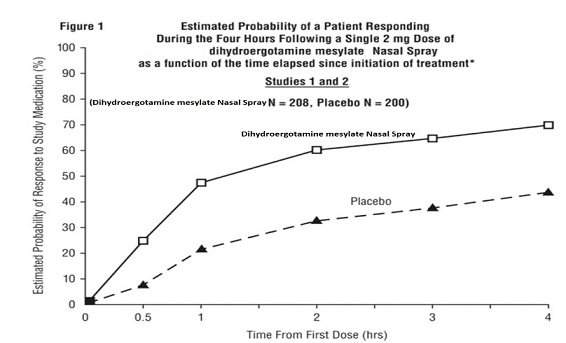
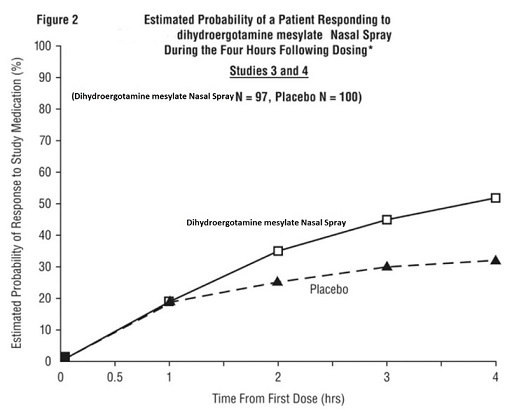
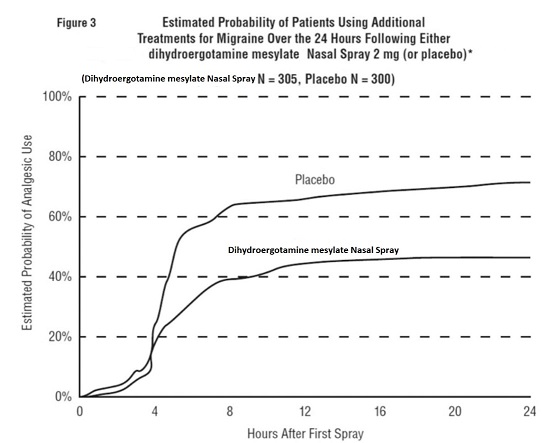
INDICATIONS AND USAGEDihydroergotamine mesylate nasal spray is indicated for the acute treatment of migraine headaches with or without aura. Dihydroergotamine mesylate nasal spray is not intended for the prophylactic ...
-
CONTRAINDICATIONSThere have been a few reports of serious adverse events associated with the coadministration of dihydroergotamine and potent CYP 3A4 inhibitors, such as protease inhibitors and macrolide ...
-
WARNINGSDihydroergotamine mesylate nasal spray should only be used where a clear diagnosis of migraine headache has been established. CYP 3A4 Inhibitors (e.g. Macrolide Antibiotics and Protease ...
-
PRECAUTIONSGeneral - Dihydroergotamine mesylate nasal spray may cause coronary artery vasospasm; patients who experience signs or symptoms suggestive of angina following its administration should, therefore ...
-
Drug InteractionsVasoconstrictors - Dihydroergotamine mesylate Nasal Spray should not be used with peripheral vasoconstrictors because the combination may cause synergistic elevation of blood ...
-
ADVERSE REACTIONSDuring clinical studies and the foreign postmarketing experience with dihydroergotamine mesylate nasal spray there have been no fatalities due to cardiac events. Serious cardiac events ...
-
DRUG ABUSE AND DEPENDENCECurrently available data have not demonstrated drug abuse or psychological dependence with dihydroergotamine. However, cases of drug abuse and psychological dependence in patients on other forms ...
-
OVERDOSAGETo date, there have been no reports of acute overdosage with this drug. Due to the risk of vascular spasm, exceeding the recommended dosages of dihydroergotamine mesylate nasal spray is to be ...
-
DOSAGE AND ADMINISTRATIONThe solution used in dihydroergotamine mesylate nasal spray (4 mg/mL) is intended for intranasal use and must not be injected. In clinical trials, dihydroergotamine mesylate nasal spray has been ...
-
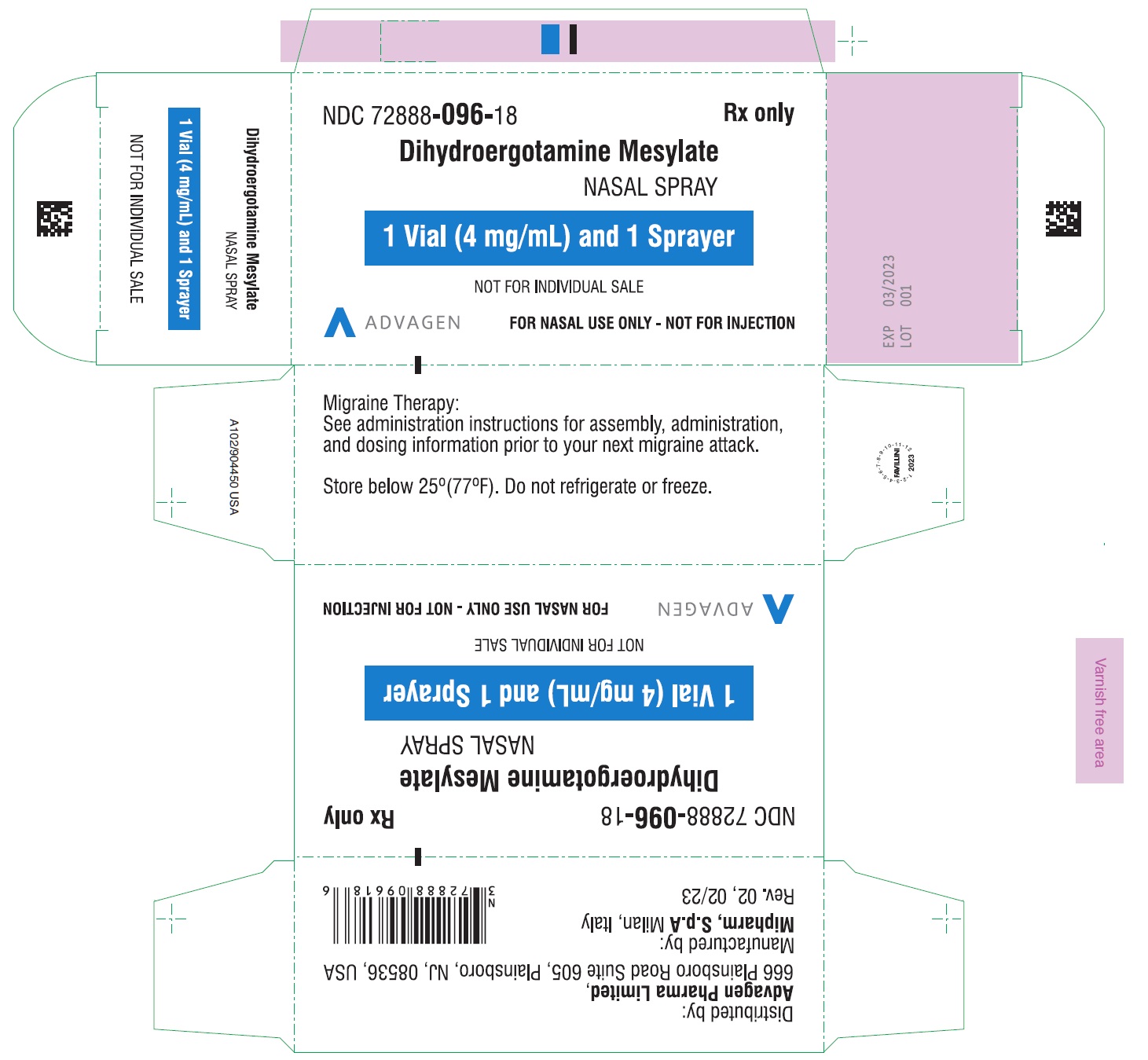
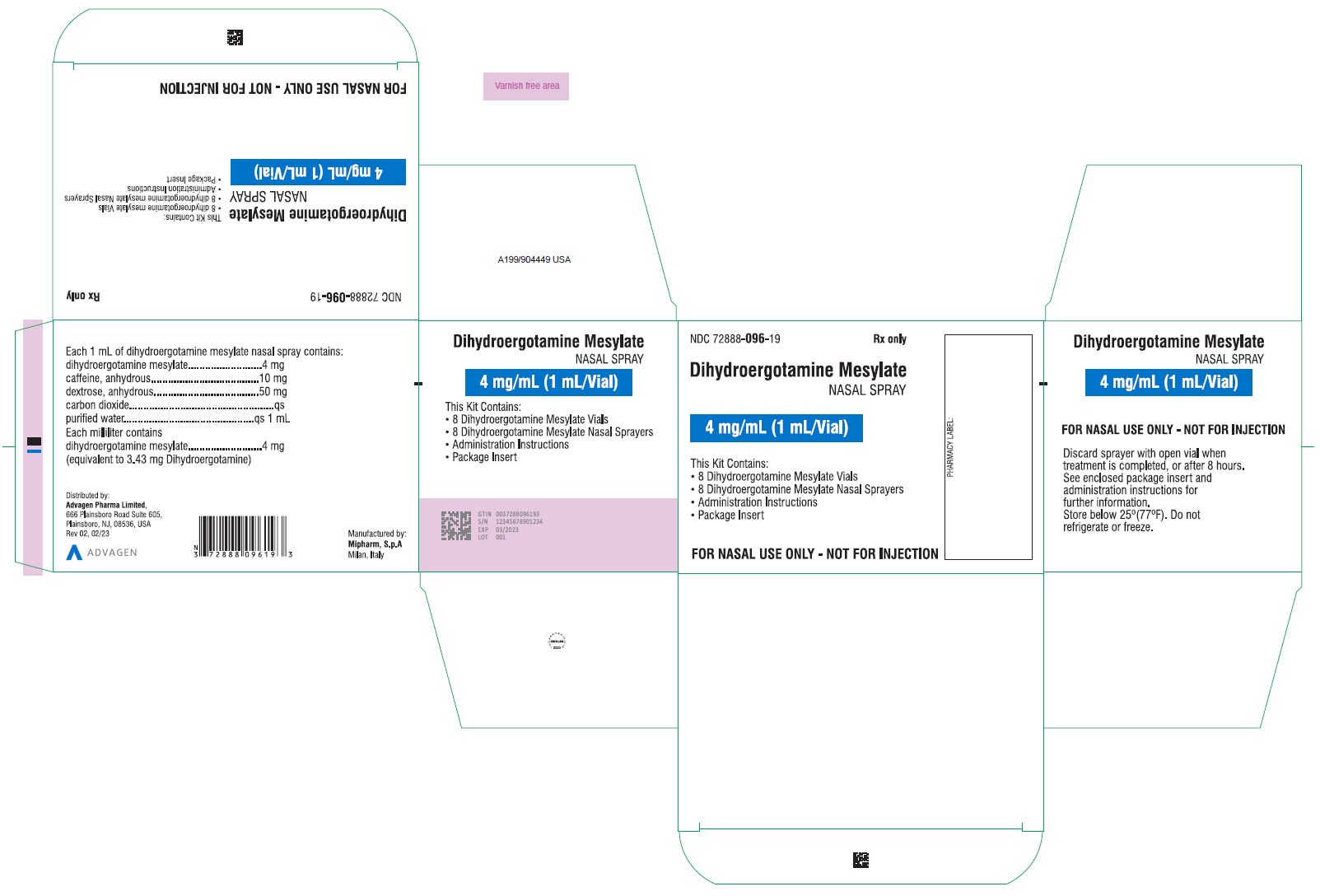
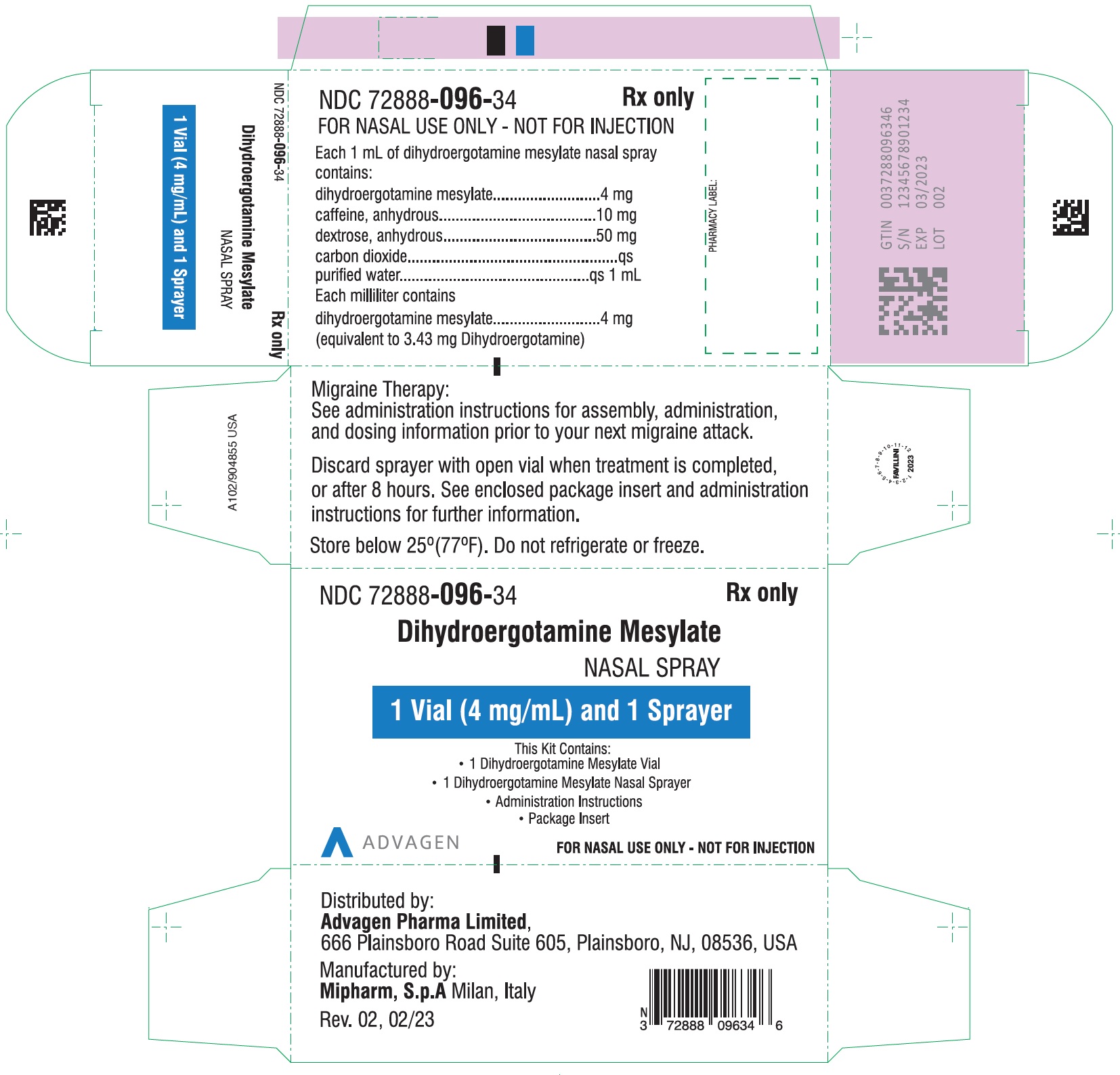
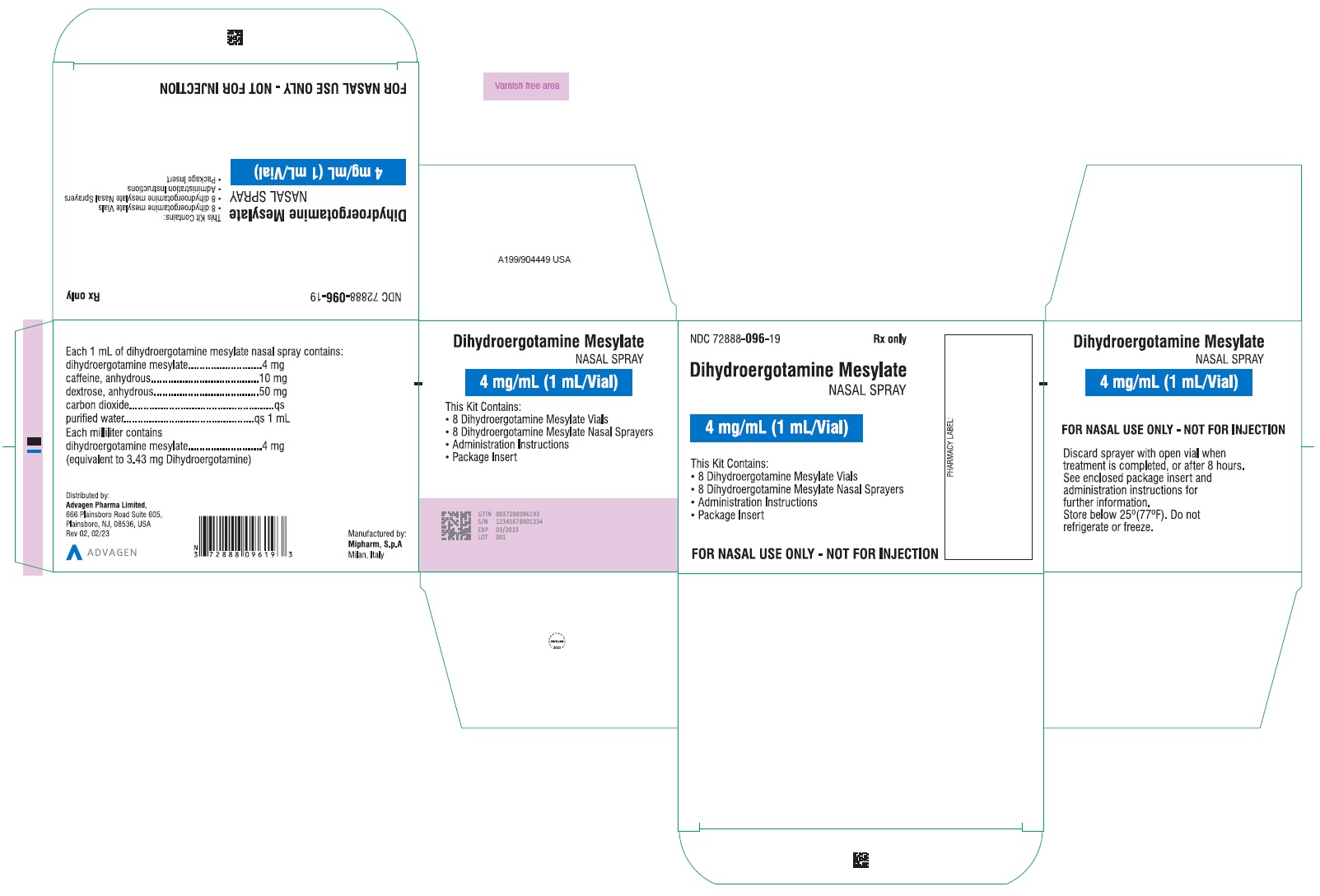
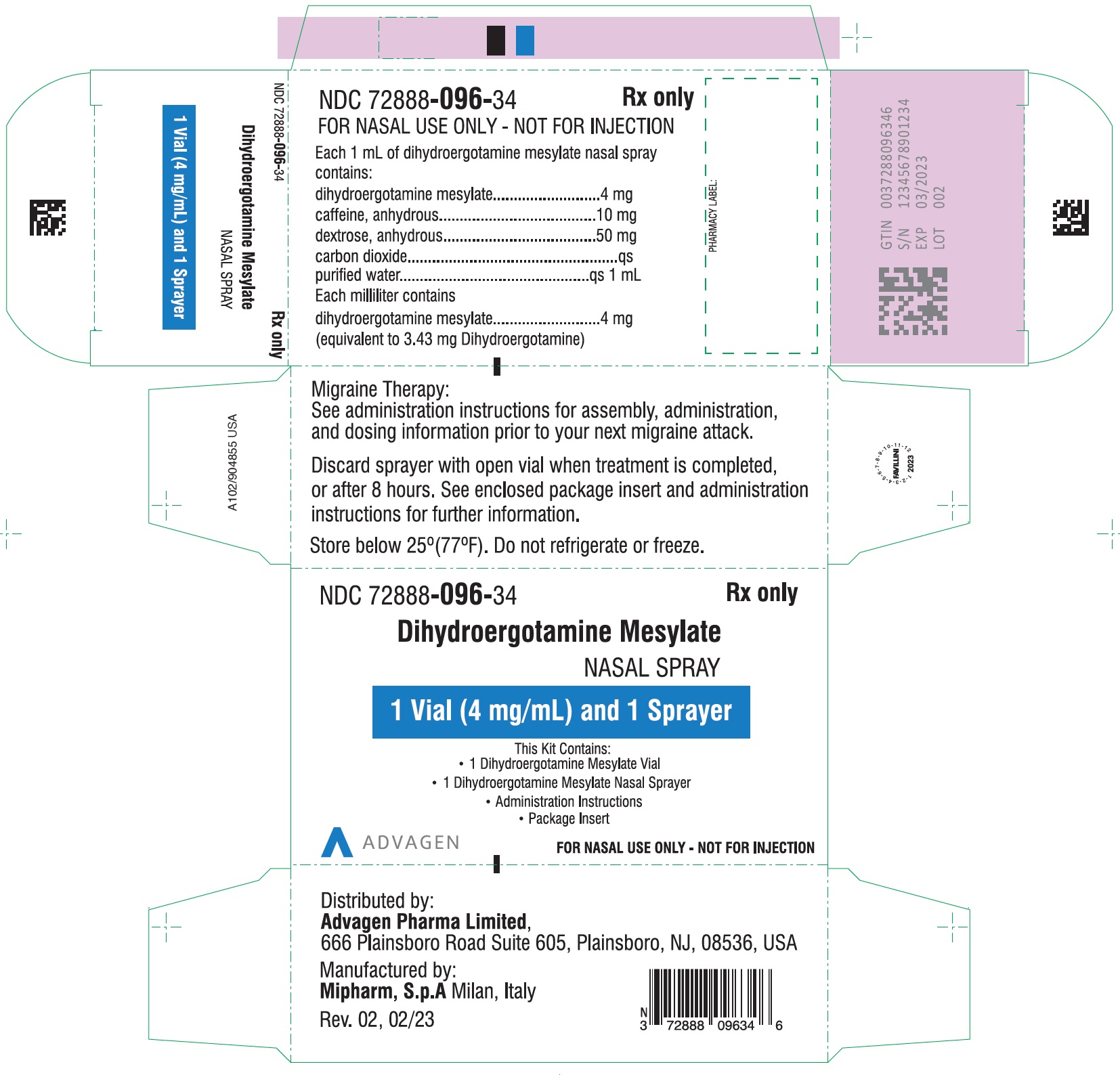
HOW SUPPLIEDDihydroergotamine mesylate nasal spray is available (as a clear, colorless to light yellow aqueous solution) in 3.5 mL amber glass vials containing 4 mg of dihydroergotamine ...
-
Patient InformationInformation for the Patient - Dihydroergotamine mesylate nasal spray. The solution used in dihydroergotamine mesylate Nasal Spray (4 mg/mL) is intended for intranasal use and must not be ...
-
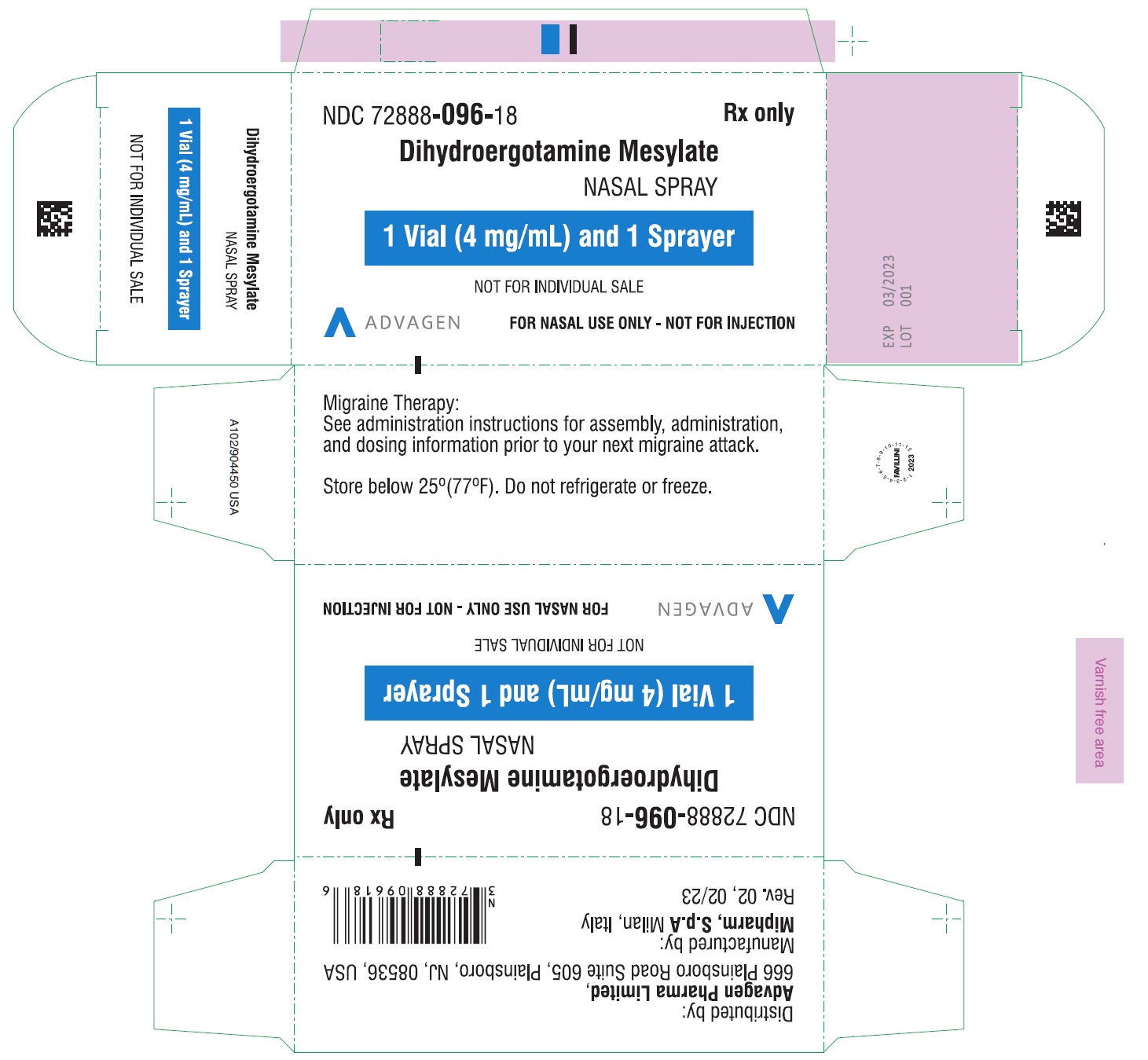
PACKAGE LABEL-PRINCIPAL DISPLAY PANELDihydroergotamine Mesylate Nasal Spray - NDC 72888-096-18 - 1 Unit (1mL Vial and 1 Sprayer) in 1 Carton Label - Dihydroergotamine Mesylate Nasal Spray - NDC 72888-096-18 - 1mL Vial Container ...
-
INGREDIENTS AND APPEARANCEProduct Information