Label: SUMATRIPTAN SUCCINATE injection
- NDC Code(s): 72603-141-01, 72603-141-02
- Packager: NorthStar Rx LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use SUMATRIPTAN INJECTION safely and effectively. See full prescribing information for SUMATRIPTAN INJECTION. SUMATRIPTAN ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGESumatriptan injection is indicated in adults for (1) the acute treatment of migraine, with or without aura, and (2) the acute treatment of cluster headache. Limitations of Use: Use only if a ...
-
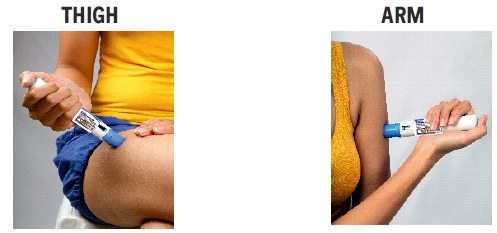
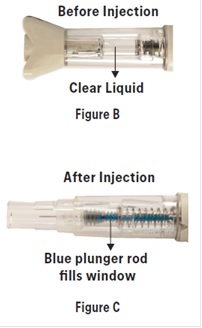
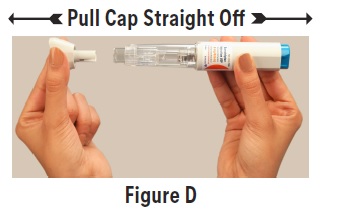
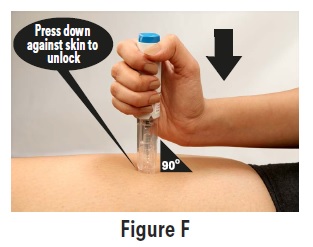
2 DOSAGE AND ADMINISTRATION2.1 Dosing Information - The maximum single recommended adult dose of sumatriptan injection for the acute treatment of migraine or cluster headache is 6 mg injected subcutaneously. For the ...
-
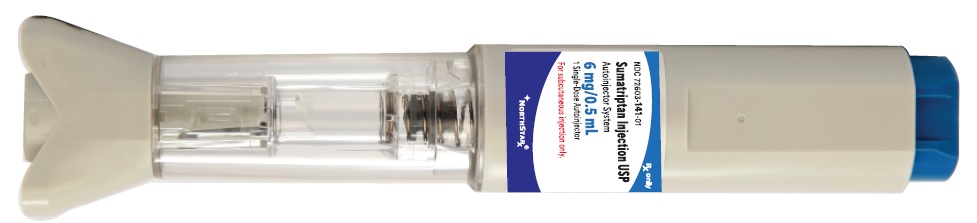
3 DOSAGE FORMS AND STRENGTHSInjection: 6 mg prefilled syringe assembled in an Autoinjector. Each 0.5 mL injection contains 8.4 mg of sumatriptan succinate, USP equivalent to 6 mg of sumatriptan.
-
4 CONTRAINDICATIONSSumatriptan injection is contraindicated in patients with: Ischemic coronary artery disease (CAD) (angina pectoris, history of myocardial infarction, or documented silent ischemia) or coronary ...
-
5 WARNINGS AND PRECAUTIONS5.1 Myocardial Ischemia, Myocardial Infarction, and Prinzmetal’s Angina - The use of sumatriptan injection is contraindicated in patients with ischemic or vasospastic CAD. There have been ...
-
6 ADVERSE REACTIONSThe following serious adverse reactions are described below and elsewhere in the labeling: Myocardial ischemia, myocardial infarction, and Prinzmetal’s angina [see Warnings and Precautions ...
-
7 DRUG INTERACTIONS7.1 Ergot-Containing Drugs - Ergot-containing drugs have been reported to cause prolonged vasospastic reactions. Because these effects may be additive, use of ergotamine-containing or ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Data from a prospective pregnancy exposure registry and epidemiological studies of pregnant women have not detected an increased frequency of birth defects or a ...
-
10 OVERDOSAGECoronary vasospasm was observed after intravenous administration of sumatriptan injection [see Contraindications (4)]. Overdoses would be expected from animal data (dogs at 0.1 g/kg, rats at 2 ...
-
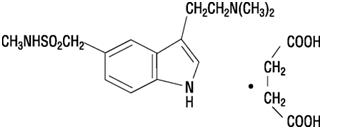
11 DESCRIPTIONSumatriptan injection, USP contains sumatriptan succinate, USP a selective 5-HT1B/1D receptor agonist. Sumatriptan succinate, USP is chemically designated as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Sumatriptan binds with high affinity to human cloned 5-HT1B/1D receptors. Sumatriptan presumably exerts its therapeutic effects in the treatment of migraine and ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - In carcinogenicity studies in mouse and rat, sumatriptan was administered orally for 78 weeks and 104 weeks ...
-
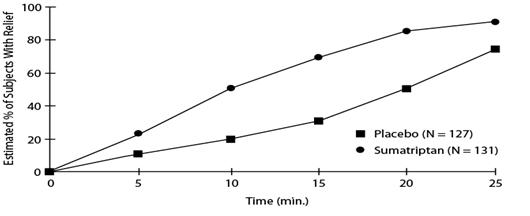
14 CLINICAL STUDIES14.1 Migraine - In controlled clinical trials enrolling more than 1,000 patients during migraine attacks who were experiencing moderate or severe pain and 1 or more of the symptoms ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGSumatriptan injection, USP contains sumatriptan (base) as the succinate salt and is supplied as a clear, colorless to pale yellow, sterile, nonpyrogenic solution as follows: NDC 72603-141-02 ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use). Risk of Myocardial Ischemia and/or Infarction, Prinzmetal’s Angina, Other ...
-
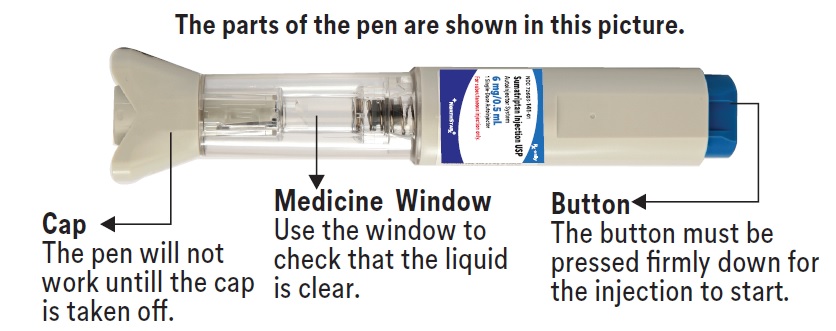
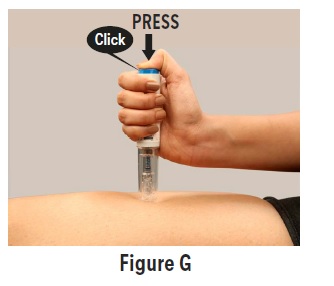
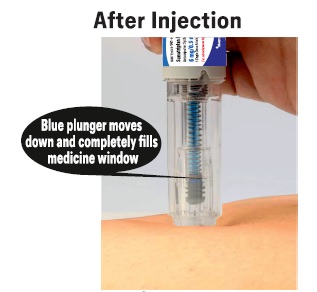
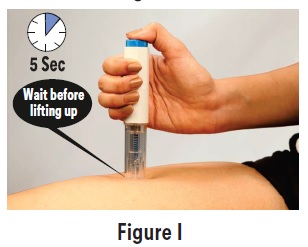
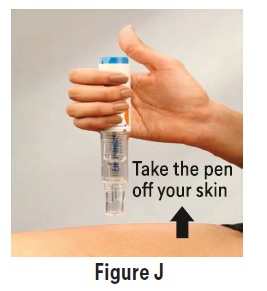
PATIENT PACKAGE INSERTPATIENT INFORMATION - Sumatriptan (soo'' ma trip' tan) Injection USP - What is the most important information I should know about sumatriptan injection? Sumatriptan can cause serious ...
-
PACKAGE LABEL PRINCIPAL DISPLAY PANEL SECTIONAutoinjector Label
-
PRINCIPAL DISPLAY PANELUnvarnished Area Consists of: 2D Barcode, Lot Number, Expiry Date and Serial Number - Autoinjector carton label
-
PRINCIPAL DISPLAY PANELBack Side Carton
-
INGREDIENTS AND APPEARANCEProduct Information







 Back Side Carton
Back Side Carton