Label: TAMSULOSIN HYDROCHLORIDE capsule
- NDC Code(s): 72603-115-01, 72603-115-02
- Packager: NorthStar Rx LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use TAMSULOSIN HYDROCHLORIDE CAPSULES safely and effectively. See full prescribing information for TAMSULOSIN HYDROCHLORIDE CAPSULES ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGETamsulosin hydrochloride capsules are indicated for the treatment of the signs and symptoms of benign prostatic hyperplasia (BPH) [see Clinical Studies (14)]. Tamsulosin hydrochloride capsules ...
-
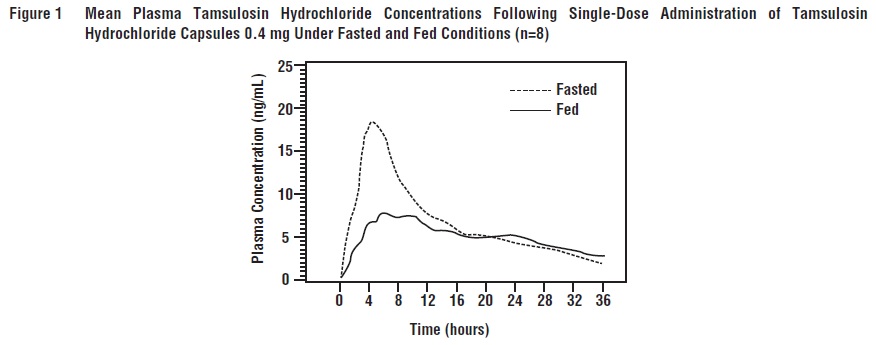
2 DOSAGE AND ADMINISTRATIONTamsulosin hydrochloride capsules 0.4 mg once daily is recommended as the dose for the treatment of the signs and symptoms of BPH. It should be administered approximately one-half hour following ...
-
3 DOSAGE FORMS AND STRENGTHSCapsule: 0.4 mg are olive green opaque/orange opaque size ‘0’ hard gelatin capsules imprinted with ‘D’ on cap and ‘53’ on body with black edible ink filled with white to off-white beadlets ...
-
4 CONTRAINDICATIONSTamsulosin hydrochloride capsules are contraindicated in patients known to be hypersensitive to tamsulosin hydrochloride or any component of tamsulosin hydrochloride capsules. Reactions have ...
-
5 WARNINGS AND PRECAUTIONS5.1 Orthostasis - The signs and symptoms of orthostasis (postural hypotension, dizziness, and vertigo) were detected more frequently in tamsulosin hydrochloride capsule-treated patients than in ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical studies are conducted under widely varying conditions, adverse reactions rates observed in the clinical studies of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Cytochrome P450 Inhibition - Strong and Moderate Inhibitors of CYP3A4 or CYP2D6 - Tamsulosin is extensively metabolized, mainly by CYP3A4 and CYP2D6. Concomitant treatment with ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - Tamsulosin hydrochloride is not indicated for use in women. There are no adequate data on the developmental risk associated with the use of tamsulosin ...
-
10 OVERDOSAGEShould overdosage of tamsulosin hydrochloride capsules lead to hypotension [see Warnings and Precautions (5.1) and Adverse Reactions (6.1)], support of the cardiovascular system is of first ...
-
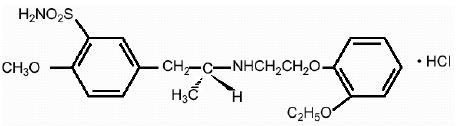
11 DESCRIPTIONTamsulosin hydrochloride is an antagonist of alpha1A adrenoceptors in the prostate. Tamsulosin hydrochloride is (-)-(R)-5-[2-[[2-(o-Ethoxyphenoxy ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The symptoms associated with benign prostatic hyperplasia (BPH) are related to bladder outlet obstruction, which is comprised of two underlying components: static and ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Rats administered doses up to 43 mg/kg/day in males and 52 mg/kg/day in females had no increases in tumor incidence, with the ...
-
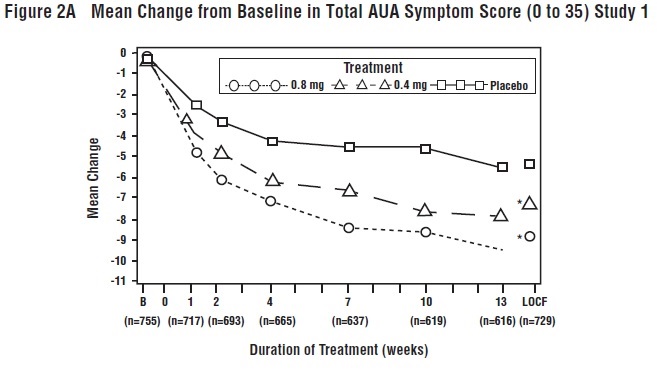
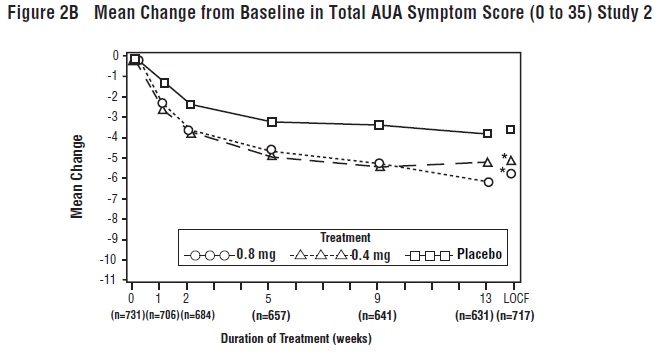
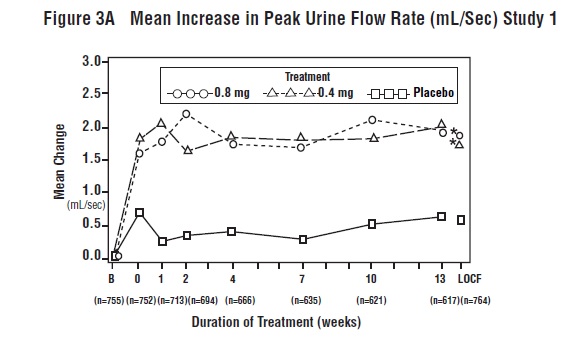
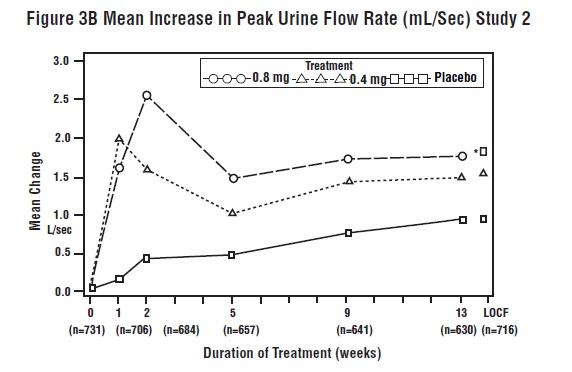
14 CLINICAL STUDIESFour placebo-controlled clinical studies and one active-controlled clinical study enrolled a total of 2296 patients (1003 received tamsulosin hydrochloride capsules 0.4 mg once daily, 491 ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGTamsulosin Hydrochloride Capsules USP, 0.4 mg are olive green opaque/orange opaque size ‘0’ hard gelatin capsules imprinted with ‘D’ on cap and ‘53’ on body with black edible ink filled with ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Patient Information) Hypotension - Advise the patient about the possible occurrence of symptoms related to postural hypotension ...
-
PATIENT INFORMATIONTamsulosin Hydrochloride Capsules, USP - (tam soo' loe sin hye" droe klor' ide) Read the Patient Information that comes with tamsulosin hydrochloride capsules before you start taking them and ...
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 0.4 mg (100 Capsules Bottle)Rx only - NDC 72603-115-01 - Namsulosin - Hydrochloride - Capsules, USP - 0.4 mg - 100 Capsules - NORTHSTAR®
-
INGREDIENTS AND APPEARANCEProduct Information