Label: ESTRADIOL tablet
- NDC Code(s): 72603-273-01, 72603-274-01, 72603-274-02, 72603-275-01, view more
- Packager: NorthStar Rx LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
BOXED WARNING
(What is this?)
BOXED WARNING
ESTROGENS INCREASE THE RISK OF ENDOMETRIAL CANCER
Close clinical surveillance of all women taking estrogens is important. Adequate diagnostic measures, including endometrial sampling when indicated, should be undertaken to rule out malignancy in all cases of undiagnosed persistent or recurring abnormal vaginal bleeding. There is no evidence that the use of “natural” estrogens results in a different endometrial risk profile than “synthetic” estrogens at equivalent estrogen doses. (See WARNINGS, Malignant neoplasms, Endometrial cancer.)
CARDIOVASCULAR AND OTHER RISKS
Estrogens with or without progestins should not be used for the prevention of cardiovascular disease. (See WARNINGS, Cardiovascular disorders.)
The Women’s Health Initiative (WHI) study reported increased risks of myocardial infarction, stroke, invasive breast cancer, pulmonary emboli, and deep vein thrombosis in postmenopausal women (50 to 79 years of age) during 5 years of treatment with oral conjugated estrogens (CE 0.625 mg) combined with medroxyprogesterone acetate (MPA 2.5 mg) relative to placebo. (See CLINICAL PHARMACOLOGY, Clinical Studies.)
The Women’s Health Initiative Memory Study (WHIMS), a substudy of WHI, reported increased risk of developing probable dementia in postmenopausal women 65 years of age or older during 4 years of treatment with oral conjugated estrogens plus medroxyprogesterone acetate relative to placebo. It is unknown whether this finding applies to younger postmenopausal women or to women taking estrogen alone therapy. (See CLINICAL PHARMACOLOGY, Clinical Studies.)
Other doses of oral conjugated estrogens with medroxyprogesterone acetate, and other combinations and dosage forms of estrogens and progestins were not studied in the WHI clinical trials and, in the absence of comparable data, these risks should be assumed to be similar. Because of these risks, estrogens with or without progestins should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman.
Close -
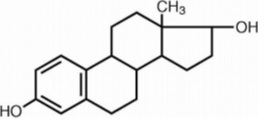
DESCRIPTIONEstradiol Tablets USP for oral administration contains 0.5, 1 or 2 mg of micronized estradiol, USP per tablet. Estradiol, USP (17β-estradiol) is a white to practically white powder, chemically ...
-
CLINICAL PHARMACOLOGYEndogenous estrogens are largely responsible for the development and maintenance of the female reproductive system and secondary sexual characteristics. Although circulating estrogens exist in a ...
-
INDICATIONS & USAGEEstradiol tablets are indicated in the: Treatment of moderate to severe vasomotor symptoms associated with the menopause. Treatment of moderate to severe symptoms of vulvar and vaginal atrophy ...
-
CONTRAINDICATIONSEstrogens should not be used in individuals with any of the following conditions: Undiagnosed abnormal genital bleeding. Known, suspected or history of cancer of the breast except in ...
-
WARNINGSSee - BOXED WARNINGS. 1. Cardiovascular disorders - Estrogen and estrogen/progestin therapy has been associated with an increased risk of cardiovascular events such as myocardial infarction ...
-
PRECAUTIONSA. General Precautions - 1. Addition of a progestin when a woman has not had a hysterectomy - Studies of the addition of a progestin for 10 or more days of a cycle of estrogen administration ...
-
ADVERSE REACTIONSSee - BOXED WARNINGS, WARNINGS, and - PRECAUTIONS. The following additional adverse reactions have been reported with estrogen and/or progestin therapy. 1. Genitourinary system - Changes ...
-
OVERDOSAGESerious ill effects have not been reported following acute ingestion of large doses of estrogen-containing oral contraceptives by young children. Overdosage of estrogen may cause nausea and ...
-
DOSAGE & ADMINISTRATIONWhen estrogen is prescribed for a postmenopausal woman with a uterus, a progestin should also be initiated to reduce the risk of endometrial cancer. A woman without a uterus does not need ...
-
HOW SUPPLIEDEstradiol Tablets, USP are available as: 0.5 mg: White to off-white, oval, flat-faced, beveled-edge, scored tablet debossed with N and 564 on the scored side and plain on the other side, packaged ...
-
PATIENT INFORMATIONEstradiol (es"tra dye' ol) Tablets, USP - Read this PATIENT INFORMATION before you start taking estradiol tablets and read what you get each time you refill estradiol tablets. There may be new ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELEstradiol Tablets, USP 0.5 mg - NDC 72603-273-01 - 100 counts - Estradiol Tablets, USP 1 mg - NDC 72603-274-01- 100 counts - Estradiol Tablets, USP 1 mg - NDC 72603-274-02 - 500 ...
-
INGREDIENTS AND APPEARANCEProduct Information