Label: ARIPIPRAZOLE tablet, orally disintegrating
-
NDC Code(s):
72578-106-06,
72578-106-30,
72578-106-78,
72578-107-06, view more72578-107-30, 72578-107-78
- Packager: Viona Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 16, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ARIPIPRAZOLE ORALLY DISINTEGRATING TABLETS safely and effectively. See full prescribing information for ARIPIPRAZOLE ORALLY ...These highlights do not include all the information needed to use ARIPIPRAZOLE ORALLY DISINTEGRATING TABLETS safely and effectively. See full prescribing information for ARIPIPRAZOLE ORALLY DISINTEGRATING TABLETS.
ARIPIPRAZOLE orally disintegrating tablets
Initial U.S. Approval: 2002WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS and SUICIDAL THOUGHTS AND BEHAVIORS WITH ANTIDEPRESSANT DRUGS
See full prescribing information for complete boxed warning.
- Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Aripiprazole is not approved for the treatment of patients with dementia-related psychosis. (5.1)
- Increased risk of suicidal thinking and behavior in children, adolescents, and young adults taking antidepressants. Monitor for worsening and emergence of suicidal thoughts and behaviors. (5.3)
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
Initial
Dose
Recommended
Dose
Maximum
Dose
Schizophrenia – adults (2.1)
10 to 15 mg/day
10 to 15 mg/day
30 mg/day
Schizophrenia – adolescents
(2.1)
2 mg/day
10 mg/day
30 mg/day
Irritability associated with autistic disorder – pediatric patients (2.4)
2 mg/day
5 to 10 mg/day
15 mg/day
Tourette's Disorder –(2.5)
Patients < 50 kg
2 mg/day
5 mg/day
10 mg/day
Patients ≥ 50 kg
2 mg/day
10 mg/day
20 mg/day
DOSAGE FORMS AND STRENGTHS
- Orally Disintegrating Tablets: 10 mg and 15 mg (3)
CONTRAINDICATIONS
- Known hypersensitivity to aripiprazole (4)
WARNINGS AND PRECAUTIONS
- Cerebrovascular Adverse Reactions in Elderly Patients with Dementia-Related Psychosis: Increased incidence of cerebrovascular adverse reactions (e.g., stroke, transient ischemic attack, including fatalities) (5.2)
- Neuroleptic Malignant Syndrome: Manage with immediate discontinuation and close monitoring (5.4)
- Tardive Dyskinesia: Discontinue if clinically appropriate (5.5)
- Metabolic Changes: Atypical antipsychotic drugs have been associated with metabolic changes that include hyperglycemia/diabetes mellitus, dyslipidemia, and body weight gain (5.6)
o Hyperglycemia/Diabetes Mellitus: Monitor glucose regularly in patients with and at risk for diabetes (5.6)
o Dyslipidemia: Undesirable alterations in lipid levels have been observed in patients treated with atypical antipsychotics (5.6)
o Weight Gain: Weight gain has been observed with atypical antipsychotic use. Monitor weight (5.6)
- Pathological Gambling and Other Compulsive Behaviors: Consider dose reduction or discontinuation (5.7)
- Orthostatic Hypotension: Monitor heart rate and blood pressure and warn patients with known cardiovascular or cerebrovascular disease, and risk of dehydration or syncope (5.8)
- Leukopenia, Neutropenia, and Agranulocytosis: have been reported with antipsychotics including aripiprazole. Patients with a history of a clinically significant low white blood cell count (WBC) or a drug-induced leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and discontinuation of aripiprazole should be considered at the first sign of a clinically significant decline in WBC in the absence of other causative factors (5.10)
- Seizures/Convulsions: Use cautiously in patients with a history of seizures or with conditions that lower the seizure threshold (5.11)
- Potential for Cognitive and Motor Impairment: Use caution when operating machinery (5.12)
- Suicide: The possibility of a suicide attempt is inherent in schizophrenia and bipolar disorder. Closely supervise high-risk patients (5.14)
ADVERSE REACTIONS
Commonly observed adverse reactions (incidence ≥ 5% and at least twice that for placebo) were (6.1):
- Adult patients with schizophrenia: akathisia
- Pediatric patients (13 to 17 years) with schizophrenia: extrapyramidal disorder, somnolence, and tremor
- Pediatric patients (6 to 17 years) with autistic disorder: sedation, fatigue, vomiting, somnolence, tremor, pyrexia, drooling, decreased appetite, salivary hypersecretion, extrapyramidal disorder, and lethargy
- Pediatric patients (6 to 18 years) with Tourette's disorder: sedation, somnolence, nausea, headache, nasopharyngitis, fatigue, increased appetite
To report SUSPECTED ADVERSE REACTIONS, contact Viona Pharmaceuticals Inc., at 1-888-304-5011 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
Dosage adjustment due to drug interactions (7.1):
Factors
Dosage Adjustments for Aripiprazole
Known CYP2D6 Poor Metabolizers
Administer half of usual dose
Known CYP2D6 Poor Metabolizers and strong
CYP3A4 inhibitors
Administer a quarter of usual dose
Strong CYP2D6 or CYP3A4 inhibitors
Administer half of usual dose
Strong CYP2D6 and CYP3A4 inhibitors
Administer a quarter of usual dose
Strong CYP3A4 inducers
Double usual dose over 1 to 2 weeks
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 1/2025
Close -
Table of ContentsTable of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS and SUICIDAL THOUGHTS AND BEHAVIORS WITH ANTIDEPRESSANT DRUGS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Schizophrenia
2.4 Irritability Associated with Autistic Disorder
2.5 Tourette’s Disorder
2.7 Dosage Adjustments for Cytochrome P450 Considerations
2.8 Dosing of Oral Solution
2.9 Dosing of Orally Disintegrating Tablets
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Increased Mortality in Elderly Patients with Dementia-Related Psychosis
5.2 Cerebrovascular Adverse Events, Including Stroke
5.3 Suicidal Thoughts and Behaviors in Children, Adolescents, and Young Adults
5.4 Neuroleptic Malignant Syndrome (NMS)
5.5 Tardive Dyskinesia
5.6 Metabolic Changes
5.7 Pathological Gambling and Other Compulsive Behaviors
5.8 Orthostatic Hypotension
5.9 Falls
5.10 Leukopenia, Neutropenia, and Agranulocytosis
5.11 Seizures/Convulsions
5.12 Potential for Cognitive and Motor Impairment
5.13 Body Temperature Regulation
5.14 Suicide
5.15 Dysphagia
5.16 Risks in Patients with Phenylketonuria
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drugs Having Clinically Important Interactions with Aripiprazole
7.2 Drugs Having No Clinically Important Interactions with Aripiprazole
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 CYP2D6 Poor Metabolizers
8.7 Hepatic and Renal Impairment
8.8 Other Specific Populations
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
10.1 Human Experience
10.2 Management of Overdosage
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Schizophrenia
14.2 Bipolar Disorder
14.4 Irritability Associated with Autistic Disorder
14.5 Tourette's Disorder
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS and SUICIDAL THOUGHTS AND BEHAVIORS WITH ANTIDEPRESSANT DRUGS
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Aripiprazole is not approved for the treatment of patients with dementia-related psychosis [see Warnings and Precautions (5.1)].
Antidepressants increased the risk of suicidal thoughts and behavior in children, adolescents, and young adults in short-term studies. These studies did not show an increase in the risk of suicidal thoughts and behavior with antidepressant use in patients over age 24; there was a reduction in risk with antidepressant use in patients aged 65 and older [see Warnings and Precautions (5.3)].
In patients of all ages who are started on antidepressant therapy, monitor closely for worsening, and for emergence of suicidal thoughts and behaviors. Advise families and caregivers of the need for close observation and communication with the prescriber [see Warnings and Precautions (5.3)].
Close -
1 INDICATIONS AND USAGEAripiprazole orally disintegrating tablets are indicated for the treatment of: Schizophrenia [see Clinical Studies (14.1)] Irritability Associated with Autistic Disorder [see Clinical Studies ...
Aripiprazole orally disintegrating tablets are indicated for the treatment of:
- Schizophrenia [see Clinical Studies (14.1)]
- Irritability Associated with Autistic Disorder [see Clinical Studies (14.4)]
- Treatment of Tourette's Disorder [see Clinical Studies (14.5)]
-
2 DOSAGE AND ADMINISTRATION2.1 Schizophrenia - Adults - The recommended starting and target dose for aripiprazole is 10 mg/day or 15 mg/day administered on a once-a-day schedule without regard to meals. Aripiprazole has ...
2.1 Schizophrenia
The recommended starting and target dose for aripiprazole is 10 mg/day or 15 mg/day administered on a once-a-day schedule without regard to meals. Aripiprazole has been systematically evaluated and shown to be effective in a dose range of 10 mg/day to 30 mg/day, when administered as the tablet formulation; however, doses higher than 10 mg/day or 15 mg/day were not more effective than 10 mg/day or 15 mg/day. Dosage increases should generally not be made before 2-weeks, the time needed to achieve steady-state [see Clinical Studies (14.1)].
Maintenance Treatment: Maintenance of efficacy in schizophrenia was demonstrated in a trial involving patients with schizophrenia who had been symptomatically stable on other antipsychotic medications for periods of 3 months or longer. These patients were discontinued from those medications and randomized to either aripiprazole 15 mg/day or placebo, and observed for relapse [see Clinical Studies (14.1)]. Patients should be periodically reassessed to determine the continued need for maintenance treatment.
Adolescents
The recommended target dose of aripiprazole is 10 mg/day. Aripiprazole was studied in adolescent patients 13 to 17 years of age with schizophrenia at daily doses of 10 mg and 30 mg. The starting daily dose of the tablet formulation in these patients was 2 mg, which was titrated to 5 mg after 2 days and to the target dose of 10 mg after 2 additional days. Subsequent dose increases should be administered in 5 mg increments. The 30 mg/day dose was not shown to be more efficacious than the 10 mg/day dose. Aripiprazole can be administered without regard to meals [see Clinical Studies (14.1)]. Patients should be periodically reassessed to determine the need for maintenance treatment.
Switching from Other Antipsychotics
There are no systematically collected data to specifically address switching patients with schizophrenia from other antipsychotics to aripiprazole or concerning concomitant administration with other antipsychotics. While immediate discontinuation of the previous antipsychotic treatment may be acceptable for some patients with schizophrenia, more gradual discontinuation may be most appropriate for others. In all cases, the period of overlapping antipsychotic administration should be minimized.
2.4 Irritability Associated with Autistic Disorder
Pediatric Patients (6 to 17 years)
The recommended dosage range for the treatment of pediatric patients with irritability associated with autistic disorder is 5 to 15 mg/day.
Dosing should be initiated at 2 mg/day. The dose should be increased to 5 mg/day, with subsequent increases to 10 or 15 mg/day if needed. Dose adjustments of up to 5 mg/day should occur gradually, at intervals of no less than 1 week [see Clinical Studies (14.4)]. Patients should be periodically reassessed to determine the continued need for maintenance treatment.
2.5 Tourette’s Disorder
Pediatric Patients (6 to 18 years)
The recommended dosage range for Tourette's Disorder is 5 to 20 mg/day.
For patients weighing less than 50 kg, dosing should be initiated at 2 mg/day with a target dose of 5 mg/day after 2 days. The dose can be increased to 10 mg/day in patients who do not achieve optimal control of tics. Dosage adjustments should occur gradually at intervals of no less than 1 week.
For patients weighing 50 kg or more, dosing should be initiated at 2 mg/day for 2 days, and then increased to 5 mg/day for 5 days, with a target dose of 10 mg/day on day 8. The dose can be increased up to 20 mg/day for patients who do not achieve optimal control of tics. Dosage adjustments should occur gradually in increments of 5 mg/day at intervals of no less than 1 week. [See Clinical Studies (14.5)].
Patients should be periodically reassessed to determine the continued need for maintenance treatment.
2.7 Dosage Adjustments for Cytochrome P450 Considerations
Dosage adjustments are recommended in patients who are known CYP2D6 poor metabolizers and in patients taking concomitant CYP3A4 inhibitors or CYP2D6 inhibitors or strong CYP3A4 inducers (see Table 2). When the coadministered drug is withdrawn from the combination therapy, aripiprazole dosage should then be adjusted to its original level. When the coadministered CYP3A4 inducer is withdrawn, aripiprazole dosage should be reduced to the original level over 1 to 2 weeks. Patients who may be receiving a combination of strong, moderate, and weak inhibitors of CYP3A4 and CYP2D6 (e.g., a strong CYP3A4 inhibitor and a moderate CYP2D6 inhibitor or a moderate CYP3A4 inhibitor with a moderate CYP2D6 inhibitor), the dosing may be reduced to one-quarter (25%) of the usual dose initially and then adjusted to achieve a favorable clinical response.
Table 2: Dose Adjustments for Aripiprazole in Patients who are known CYP2D6 Poor Metabolizers and Patients Taking Concomitant CYP2D6 Inhibitors, 3A4 Inhibitors, and/or CYP3A4 Inducers Factors
Dosage Adjustments for Aripiprazole
Known CYP2D6 Poor Metabolizers
Administer half of usual dose
Known CYP2D6 Poor Metabolizers taking concomitant strong CYP3A4 inhibitors (e.g., itraconazole, clarithromycin)
Administer a quarter of usual dose
Strong CYP2D6 (e.g., quinidine, fluoxetine, paroxetine) or CYP3A4 inhibitors (e.g., itraconazole, clarithromycin)
Administer half of usual dose
Strong CYP2D6 and CYP3A4 inhibitors
Administer a quarter of usual dose
Strong CYP3A4 inducers (e.g., carbamazepine, rifampin)
Double usual dose over 1 to 2 weeks
2.8 Dosing of Oral Solution
The oral solution can be substituted for tablets on a mg-per-mg basis up to the 25 mg dose level. Patients receiving 30 mg tablets should receive 25 mg of the solution [see Clinical Pharmacology (12.3)].
Close2.9 Dosing of Orally Disintegrating Tablets
The dosing for aripiprazole orally disintegrating tablets is the same as for the oral tablets [see Dosage and Administration (2.1) and (2.4)].
-
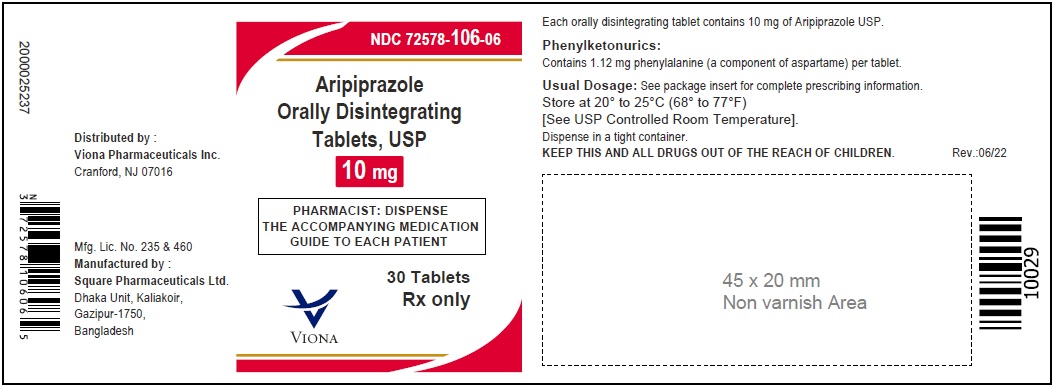
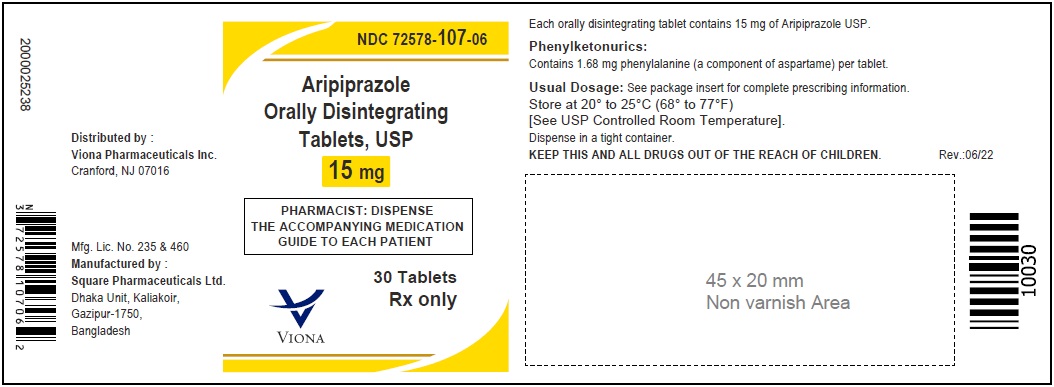
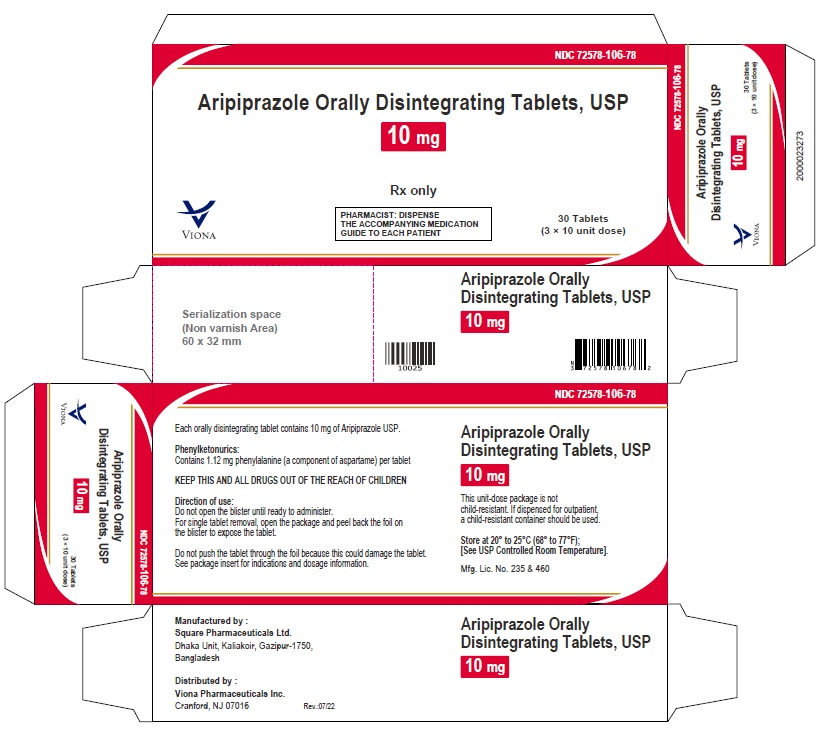
3 DOSAGE FORMS AND STRENGTHSAripiprazole orally disintegrating tablets, USP are available as described in Table 4. Table 4: Aripiprazole Orally Disintegrating Tablets Presentations - Tablet - Tablet ...
Aripiprazole orally disintegrating tablets, USP are available as described in Table 4.
CloseTable 4: Aripiprazole Orally Disintegrating Tablets Presentations Tablet
Tablet
Tablet
Strength
Color/Shape
Markings
10 mg
white to off-white
capsule-shaped
ZF 41
15 mg
white to off-white
ZF 42
round-shaped
-
4 CONTRAINDICATIONSAripiprazole is contraindicated in patients with a history of a hypersensitivity reaction to aripiprazole. Reactions have ranged from pruritus/urticaria to anaphylaxis [see Adverse Reactions ...
Aripiprazole is contraindicated in patients with a history of a hypersensitivity reaction to aripiprazole. Reactions have ranged from pruritus/urticaria to anaphylaxis [see Adverse Reactions (6.2)].
Close -
5 WARNINGS AND PRECAUTIONS5.1 Increased Mortality in Elderly Patients with Dementia-Related Psychosis - Increased Mortality - Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an ...
5.1 Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Aripiprazole is not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning].
Safety Experience in Elderly Patients with Psychosis Associated with Alzheimer's Disease
In three, 10-week, placebo-controlled studies of aripiprazole in elderly patients with psychosis associated with Alzheimer's disease (n=938; mean age: 82.4 years; range: 56-99 years), the adverse reactions that were reported at an incidence of ≥3% and aripiprazole incidence at least twice that for placebo were lethargy [placebo 2%, aripiprazole 5%], somnolence (including sedation) [placebo 3%, aripiprazole 8%], and incontinence (primarily, urinary incontinence) [placebo 1%, aripiprazole 5%], excessive salivation [placebo 0%, aripiprazole 4%], and lightheadedness [placebo 1%, aripiprazole 4%].
The safety and efficacy of aripiprazole in the treatment of patients with psychosis associated with dementia have not been established. If the prescriber elects to treat such patients with aripiprazole, assess for the emergence of difficulty swallowing or excessive somnolence, which could predispose to accidental injury or aspiration [see Boxed Warning].
5.2 Cerebrovascular Adverse Events, Including Stroke
In placebo-controlled clinical studies (two flexible dose and one fixed dose study) of dementia-related psychosis, there was an increased incidence of cerebrovascular adverse events (e.g., stroke, transient ischemic attack), including fatalities, in aripiprazole-treated patients (mean age: 84 years; range: 78 to 88 years). In the fixed-dose study, there was a statistically significant dose response relationship for cerebrovascular adverse events in patients treated with aripiprazole. Aripiprazole is not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning].
5.3 Suicidal Thoughts and Behaviors in Children, Adolescents, and Young Adults
Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term, placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18 - 24) with MDD and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
The pooled analyses of placebo-controlled trials in children and adolescents with MDD, Obsessive Compulsive Disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs. placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1000 patients treated) are provided in Table 5.
Table 5: Age Range
Drug-Placebo Difference in Number of Cases of Suicidality per 1000 Patients Treated
Increases Compared to Placebo
<18
18 - 24
14 additional cases
5 additional cases
Decreases Compared to Placebo
25 - 64
≥65
1 fewer case
6 fewer cases
No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about drug effect on suicide.
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for MDD as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient's presenting symptoms.
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to healthcare providers. Such monitoring should include daily observation by families and caregivers. Prescriptions for aripiprazole should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose.
Screening Patients for Bipolar Disorder: A major depressive episode may be the initial presentation of bipolar disorder. It is generally believed (though not established in controlled trials) that treating such an episode with an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder. Whether any of the symptoms described above represent such a conversion is unknown. However, prior to initiating treatment with an antidepressant, patients with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression.
It should be noted that aripiprazole is not approved for use in treating depression in the pediatric population.
5.4 Neuroleptic Malignant Syndrome (NMS)
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) may occur with administration of antipsychotic drugs, including aripiprazole. Rare cases of NMS occurred during aripiprazole treatment in the worldwide clinical database. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia). Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure.
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to exclude cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever, and primary central nervous system pathology.
The management of NMS should include: 1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy; 2) intensive symptomatic treatment and medical monitoring; and 3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for uncomplicated NMS.
If a patient requires antipsychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. The patient should be carefully monitored, since recurrences of NMS have been reported.
5.5 Tardive Dyskinesia
A syndrome of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with antipsychotic drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of antipsychotic treatment, which patients are likely to develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
The risk of developing tardive dyskinesia and the likelihood that it will become irreversible are believed to increase as the duration of treatment and the total cumulative dose of antipsychotic drugs administered to the patient increase. However, the syndrome can develop, although much less commonly, after relatively brief treatment periods at low doses.
Tardive dyskinesia may remit, partially or completely, if antipsychotic treatment is withdrawn. Antipsychotic treatment, itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome and, thereby, may possibly mask the underlying process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown.
Given these considerations, aripiprazole should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients who suffer from a chronic illness that (1) is known to respond to antipsychotic drugs and (2) for whom alternative, equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be reassessed periodically.
If signs and symptoms of tardive dyskinesia appear in a patient on aripiprazole, drug discontinuation should be considered. However, some patients may require treatment with aripiprazole despite the presence of the syndrome.
5.6 Metabolic Changes
Atypical antipsychotic drugs have been associated with metabolic changes that include hyperglycemia/diabetes mellitus, dyslipidemia, and body weight gain. While all drugs in the class have been shown to produce some metabolic changes, each drug has its own specific risk profile.
Hyperglycemia/Diabetes Mellitus
Hyperglycemia, in some cases extreme and associated with ketoacidosis or hyperosmolar coma or death, has been reported in patients treated with atypical antipsychotics. There have been reports of hyperglycemia in patients treated with aripiprazole [see Adverse Reactions (6.1, 6.2)]. Assessment of the relationship between atypical antipsychotic use and glucose abnormalities is complicated by the possibility of an increased background risk of diabetes mellitus in patients with schizophrenia and the increasing incidence of diabetes mellitus in the general population. Given these confounders, the relationship between atypical antipsychotic use and hyperglycemia-related adverse events is not completely understood. However, epidemiological studies suggest an increased risk of hyperglycemia-related adverse reactions in patients treated with the atypical antipsychotics. Because aripiprazole was not marketed at the time these studies were performed, it is not known if aripiprazole is associated with this increased risk. Precise risk estimates for hyperglycemia-related adverse reactions in patients treated with atypical antipsychotics are not available.
Patients with an established diagnosis of diabetes mellitus who are started on atypical antipsychotics should be monitored regularly for worsening of glucose control. Patients with risk factors for diabetes mellitus (e.g., obesity, family history of diabetes) who are starting treatment with atypical antipsychotics should undergo fasting blood glucose testing at the beginning of treatment and periodically during treatment. Any patient treated with atypical antipsychotics should be monitored for symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness. Patients who develop symptoms of hyperglycemia during treatment with atypical antipsychotics should undergo fasting blood glucose testing. In some cases, hyperglycemia has resolved when the atypical antipsychotic was discontinued; however, some patients required continuation of anti-diabetic treatment despite discontinuation of the suspect drug.
Adults
In an analysis of 13 placebo-controlled monotherapy trials in adults, primarily with schizophrenia or another disorder, the mean change in fasting glucose in aripiprazole-treated patients (+4.4 mg/dL; median exposure 25 days; N=1057) was not significantly different than in placebo-treated patients (+2.5 mg/dL; median exposure 22 days; N=799). Table 6 shows the proportion of aripiprazole-treated patients with normal and borderline fasting glucose at baseline (median exposure 25 days) that had treatment-emergent high fasting glucose measurements compared to placebo-treated patients (median exposure 22 days).
Table 6: Changes in Fasting Glucose From Placebo-Controlled Monotherapy Trials in Adult Patients Category Change (at least once) from Baseline
Treatment Arm
n/N
%
Fasting Glucose
Normal to High
(<100 mg/dL to ≥126 mg/dL)
Aripiprazole
31/822
3.8
Placebo
22/605
3.6
Borderline to High
(≥100 mg/dL and <126 mg/dL to ≥126 mg/dL)
Aripiprazole
31/176
17.6
Placebo
13/142
9.2
At 24-weeks, the mean change in fasting glucose in aripiprazole-treated patients was not significantly different than in placebo-treated patients [+2.2 mg/dL (n=42) and +9.6 mg/dL (n=28), respectively].
Pediatric Patients and Adolescents
In an analysis of two placebo-controlled trials in adolescents with schizophrenia (13 to 17 years) and pediatric patients with another disorder (10 to 17 years), the mean change in fasting glucose in aripiprazole-treated patients (+4.8 mg/dL; with a median exposure of 43 days; N=259) was not significantly different than in placebo-treated patients (+1.7 mg/dL; with a median exposure of 42 days; N=123).
In an analysis of two placebo-controlled trials in pediatric and adolescent patients with irritability associated with autistic disorder (6 to 17 years) with median exposure of 56 days, the mean change in fasting glucose in aripiprazole-treated patients (–0.2 mg/dL; N=83) was not significantly different than in placebo-treated patients (–0.6 mg/dL; N=33).
In an analysis of two placebo-controlled trials in pediatric and adolescent patients with Tourette's disorder (6 to 18 years) with median exposure of 57 days, the mean change in fasting glucose in Aripiprazole-treated patients (0.79 mg/dL; N=90) was not significantly different than in placebo-treated patients (–1.66 mg/dL; N=58).
Table 8 shows the proportion of patients with changes in fasting glucose levels from the pooled adolescent schizophrenia and another indication (median exposure of 42-43 days), from two placebo-controlled trials in pediatric patients (6 to 17 years) with irritability associated with autistic disorder (median exposure of 56 days), and from the two placebo-controlled trials in pediatric patients (6 to 18 year) with Tourette's Disorder (median exposure 57 days).
Table 8: Changes in Fasting Glucose From Placebo-Controlled Trials in Pediatric and Adolescent Patients Category Change (at least once) from Baseline
Indication
Treatment Arm
n/N
%
Fasting Glucose
Normal to High
(<100 mg/dL to ≥ 126 mg/dL)
Pooled Schizophrenia and Another Disorder
Aripiprazole
2/236
0.8
Placebo
2/110
1.8
Irritability Associated with Autistic Disorder
Aripiprazole
0/73
0
Placebo
0/32
0
Tourette's Disorder
Aripiprazole
3/88
3.4
Placebo
1/58
1.7
Fasting Glucose Borderline to High
(≥ 100 mg/dL and <126 mg/dL to ≥ 126 mg/dL)
Pooled Schizophrenia and Another Disorder
Aripiprazole
1/22
4.5
Placebo
0/12
0
Irritability Associated with Autistic Disorder
Aripiprazole
0/9
0
Placebo
0/1
0
Tourette's Disorder
Aripiprazole
0/11
0
Placebo
0/4
0
At 12-weeks in the pooled adolescent schizophrenia and other pediatric disorder trials, the mean change in fasting glucose in aripiprazole-treated patients was not significantly different than in placebo-treated patients [+2.4 mg/dL (n=81) and +0.1 mg/dL (n=15), respectively].
Dyslipidemia
Undesirable alterations in lipids have been observed in patients treated with atypical antipsychotics.
There were no significant differences between aripiprazole- and placebo-treated patients in the proportion with changes from normal to clinically significant levels for fasting/nonfasting total cholesterol, fasting triglycerides, fasting LDLs, and fasting/nonfasting HDLs. Analyses of patients with at least 12 or 24 weeks of exposure were limited by small numbers of patients.
Adults
Table 9 shows the proportion of adult patients, primarily from pooled schizophrenia and other monotherapy placebo-controlled trials, with changes in total cholesterol (pooled from 17 trials; median exposure 21 to 25 days), fasting triglycerides (pooled from eight trials; median exposure 42 days), fasting LDL cholesterol (pooled from eight trials; median exposure 39 to 45 days, except for placebo-treated patients with baseline normal fasting LDL measurements, who had median treatment exposure of 24 days) and HDL cholesterol (pooled from nine trials; median exposure 40 to 42 days).
Table 9: Changes in Blood Lipid Parameters from Placebo-Controlled Monotherapy Trials in Adults Treatment Arm
n/N
%
Total Cholesterol
Normal to High
(<200 mg/dL to ≥240 mg/dL)
Aripiprazole
34/1357
2.5
Placebo
27/973
2.8
Fasting Triglycerides
Normal to High
(<150 mg/dL to ≥200 mg/dL)
Aripiprazole
40/539
7.4
Placebo
30/431
7
Fasting LDL Cholesterol Normal to High
(<100 mg/dL to ≥160 mg/dL)
Aripiprazole
2/332
0.6
Placebo
2/268
0.7
HDL Cholesterol
Normal to Low
(≥40 mg/dL to <40 mg/dL)
Aripiprazole
121/1066
11.4
Placebo
99/794
12.5
In monotherapy trials in adults, the proportion of patients at 12 weeks and 24 weeks with changes from Normal to High in total cholesterol (fasting/nonfasting), fasting triglycerides, and fasting LDL cholesterol were similar between aripiprazole- and placebo-treated patients: at 12 weeks, Total Cholesterol (fasting/nonfasting), 1/71 (1.4%) vs. 3/74 (4.1%); Fasting Triglycerides, 8/62 (12.9%) vs. 5/37 (13.5%); Fasting LDL Cholesterol, 0/34 (0%) vs. 1/25 (4.0%), respectively; and at 24 weeks, Total Cholesterol (fasting/nonfasting), 1/42 (2.4%) vs. 3/37 (8.1%); Fasting Triglycerides, 5/34 (14.7%) vs. 5/20 (25%); Fasting LDL Cholesterol, 0/22 (0%) vs. 1/18 (5.6%), respectively.
Pediatric Patients and Adolescents
Table 11 shows the proportion of adolescents with schizophrenia (13 to 17 years) and pediatric patients with another disorder (10 to 17 years) with changes in total cholesterol and HDL cholesterol (pooled from two placebo-controlled trials; median exposure 42 to 43 days) and fasting triglycerides (pooled from two placebo-controlled trials; median exposure 42 to 44 days).
Table 11: Changes in Blood Lipid Parameters From Placebo-Controlled Monotherapy Trials in Pediatric and Adolescent Patients in Schizophrenia and Another Disorder Treatment Arm
n/N
%
Total Cholesterol
Normal to High
(<170 mg/dL to ≥200 mg/dL)
Aripiprazole
3/220
1.4
Placebo
0/116
0
Fasting Triglycerides
Normal to High
(<150 mg/dL to ≥200 mg/dL)
Aripiprazole
7/187
3.7
Placebo
4/85
4.7
HDL Cholesterol
Normal to Low
(≥40 mg/dL to <40 mg/dL)
Aripiprazole
27/236
11.4
Placebo
22/109
20.2
In monotherapy trials of adolescents with schizophrenia and pediatric patients with another disorder, the proportion of patients at 12 weeks and 24 weeks with changes from Normal to High in total cholesterol (fasting/nonfasting), fasting triglycerides, and fasting LDL cholesterol were similar between aripiprazole- and placebo-treated patients: at 12 weeks, Total Cholesterol (fasting/nonfasting), 0/57 (0%) vs. 0/15 (0%); Fasting Triglycerides, 2/72 (2.8%) vs. 1/14 (7.1%), respectively; and at 24 weeks, Total Cholesterol (fasting/nonfasting), 0/36 (0%) vs. 0/12 (0%); Fasting Triglycerides, 1/47 (2.1%) vs. 1/10 (10%), respectively.
Table 12 shows the proportion of patients with changes in total cholesterol (fasting/nonfasting) and fasting triglycerides (median exposure 56 days) and HDL cholesterol (median exposure 55 to 56 days) from two placebo-controlled trials in pediatric patients (6 to 17 years) with irritability associated with autistic disorder.
Table 12: Changes in Blood Lipid Parameters From Placebo-Controlled Trials in Pediatric Patients with Autistic Disorder Treatment Arm
n/N
%
Total Cholesterol
Normal to High
(<170 mg/dL to ≥200 mg/dL)
Aripiprazole
1/95
1.1
Placebo
0/34
0
Fasting Triglycerides
Normal to High
(<150 mg/dL to ≥200 mg/dL)
Aripiprazole
0/75
0
Placebo
0/30
0
HDL Cholesterol
Normal to Low
(≥40 mg/dL to <40 mg/dL)
Aripiprazole
9/107
8.4
Placebo
5/49
10.2
Table 13 shows the proportion of patients with changes in total cholesterol (fasting/nonfasting) and fasting triglycerides (median exposure 57 days) and HDL cholesterol (median exposure 57 days) from two placebo-controlled trials in pediatric patients (6 to 18 years) with Tourette's Disorder.
Table 13: Changes in Blood Lipid Parameters From Placebo-Controlled Trials in Pediatric Patients with Tourette's Disorder Treatment Arm
n/N
%
Total Cholesterol
Normal to High
(<170 mg/dL to ≥200 mg/dL)
Aripiprazole
1/85
1.2
Placebo
0/46
0
Fasting Triglycerides
Normal to High
(<150 mg/dL to ≥200 mg/dL)
Aripiprazole
5/94
5.3
Placebo
2/55
3.6
HDL Cholesterol
Normal to Low
(≥40 mg/dL to <40 mg/dL)
Aripiprazole
4/108
3.7
Placebo
2/67
3.0
Weight gain has been observed with atypical antipsychotic use. Clinical monitoring of weight is recommended.
Adults
In an analysis of 13 placebo-controlled monotherapy trials, primarily from pooled schizophrenia and another disorder, with a median exposure of 21 to 25 days, the mean change in body weight in aripiprazole-treated patients was +0.3 kg (N=1673) compared to –0.1 kg (N=1100) in placebo-controlled patients. At 24 weeks, the mean change from baseline in body weight in aripiprazole-treated patients was –1.5 kg (n=73) compared to –0.2 kg (n=46) in placebo-treated patients.
Table 14 shows the percentage of adult patients with weight gain ≥7% of body weight by indication.
Table 14: Percentage of Patients From Placebo-Controlled Trials in Adult Patients with Weight Gain ≥7% of Body Weight - *
- 4 to 6 weeks duration,† 3 weeks duration
Weight Gain ≥7% of Body Weight
Indication
Treatment Arm
N
Patients
n (%)
Schizophrenia*
Aripiprazole
852
69 (8.1)
Placebo
379
12 (3.2)
Another Disorder †
Aripiprazole
719
16 (2.2)
Placebo
598
16 (2.7)
Pediatric Patients and Adolescents
In an analysis of two placebo-controlled trials in adolescents with schizophrenia (13 to 17 years) and pediatric patients with another disorder (10 to 17 years) with median exposure of 42 to 43 days, the mean change in body weight in aripiprazole-treated patients was +1.6 kg (N=381) compared to +0.3 kg (N=187) in placebo-treated patients. At 24 weeks, the mean change from baseline in body weight in aripiprazole-treated patients was +5.8 kg (n=62) compared to +1.4 kg (n=13) in placebo-treated patients.
In two short-term, placebo-controlled trials in patients (6 to 17 years) with irritability associated with autistic disorder with median exposure of 56 days, the mean change in body weight in aripiprazole-treated patients was +1.6 kg (n=209) compared to +0.4 kg (n=98) in placebo-treated patients.
In two short-term, placebo-controlled trials in patients (6 to 18 years) with Tourette's Disorder with median exposure of 57 days, the mean change in body weight in Aripiprazole-treated patients was +1.5 kg (n=105) compared to +0.4 kg (n=66) in placebo-treated patients.
Table 15 shows the percentage of pediatric and adolescent patients with weight gain ≥7% of body weight by indication.
Table 15: Percentage of Patients From Placebo-Controlled Monotherapy Trials in Pediatric and Adolescent Patients with Weight Gain ≥7% of Body Weight Weight Gain ≥7% of Body Weight
Indication
Treatment Arm
N
Patients
n (%)
Pooled Schizophrenia and Another Disorder*
Aripiprazole
381
20 (5.2)
Placebo
187
3 (1.6)
Irritability Associated with Autistic Disorder†
Aripiprazole
209
55 (26.3)
Placebo
98
7 (7.1)
Tourette's Disorder‡
Aripiprazole
105
21 (20.0)
Placebo
66
5 (7.6)
In an open-label trial that enrolled patients from the two placebo-controlled trials of adolescents with schizophrenia (13 to 17 years) and pediatric patients with another disorder (10 to 17 years), 73.2% of patients (238/325) completed 26 weeks of therapy with aripiprazole. After 26-weeks, 32.8% of patients gained ≥7% of their body weight, not adjusted for normal growth. To adjust for normal growth, z-scores were derived (measured in standard deviations [SD]), which normalize for the natural growth of pediatric patients and adolescents by comparisons to age- and gender-matched population standards. A z-score change <0.5 SD is considered not clinically significant. After 26 weeks, the mean change in z-score was 0.09 SD.
In an open-label trial that enrolled patients from two short-term, placebo-controlled trials, patients (6 to 17 years) with irritability associated with autistic disorder, as well as de novo patients, 60.3% (199/330) completed one year of therapy with aripiprazole. The mean change in weight z-score was 0.26 SDs for patients receiving >9 months of treatment.
When treating pediatric patients for any indication, weight gain should be monitored and assessed against that expected for normal growth.
5.7 Pathological Gambling and Other Compulsive Behaviors
Post-marketing case reports suggest that patients can experience intense urges, particularly for gambling, and the inability to control these urges while taking aripiprazole. Other compulsive urges, reported less frequently, include: sexual urges, shopping, eating or binge eating, and other impulsive or compulsive behaviors. Because patients may not recognize these behaviors as abnormal, it is important for prescribers to ask patients or their caregivers specifically about the development of new or intense gambling urges, compulsive sexual urges, compulsive shopping, binge or compulsive eating, or other urges while being treated with aripiprazole. It should be noted that impulse-control symptoms can be associated with the underlying disorder. In some cases, although not all, urges were reported to have stopped when the dose was reduced or the medication was discontinued. Compulsive behaviors may result in harm to the patient and others if not recognized. Consider dose reduction or stopping the medication if a patient develops such urges.
5.8 Orthostatic Hypotension
Aripiprazole may cause orthostatic hypotension, perhaps due to its α1-adrenergic receptor antagonism. The incidence of orthostatic hypotension-associated events from short-term, placebo-controlled trials of adult patients on oral aripiprazole (n=2467) included (aripiprazole incidence, placebo incidence) orthostatic hypotension (1%, 0.3%), postural dizziness (0.5%, 0.3%), and syncope (0.5%, 0.4%); and of pediatric patients 6 to 18 years of age (n=732) on oral aripiprazole included orthostatic hypotension (0.5%, 0%), postural dizziness (0.4%, 0 %), and syncope (0.2%, 0%) [see Adverse Reactions (6.1)].
The incidence of a significant orthostatic change in blood pressure (defined as a decrease in systolic blood pressure ≥20 mmHg accompanied by an increase in heart rate ≥25 bpm when comparing standing to supine values) for aripiprazole was not meaningfully different from placebo (aripiprazole incidence, placebo incidence): in adult oral aripiprazole-treated patients (4%, 2%), in pediatric oral aripiprazole-treated patients aged 6 to 18 years (0.4%, 1%).
Aripiprazole should be used with caution in patients with known cardiovascular disease (history of myocardial infarction or ischemic heart disease, heart failure or conduction abnormalities), cerebrovascular disease, or conditions which would predispose patients to hypotension (dehydration, hypovolemia, and treatment with antihypertensive medications) [see Drug Interactions (7.1)].
5.9 Falls
Antipsychotics, including aripiprazole, may cause somnolence, postural hypotension, motor and sensory instability, which may lead to falls and, consequently, fractures or other injuries. For patients with diseases, conditions, or medications that could exacerbate these effects, complete fall risk assessments when initiating antipsychotic treatment and recurrently for patients on long-term antipsychotic therapy.
5.10 Leukopenia, Neutropenia, and Agranulocytosis
In clinical trials and/or postmarketing experience, events of leukopenia and neutropenia have been reported temporally related to antipsychotic agents, including aripiprazole. Agranulocytosis has also been reported.
Possible risk factors for leukopenia/neutropenia include pre-existing low white blood cell count (WBC)/absolute neutrophil count (ANC) and history of drug-induced leukopenia/neutropenia. In patients with a history of a clinically significant low WBC/ANC or drug-induced leukopenia/neutropenia, perform a complete blood count (CBC) frequently during the first few months of therapy. In such patients, consider discontinuation of aripiprazole at the first sign of a clinically significant decline in WBC in the absence of other causative factors.
Monitor patients with clinically significant neutropenia for fever or other symptoms or signs of infection and treat promptly if such symptoms or signs occur. Discontinue aripiprazole in patients with severe neutropenia (absolute neutrophil count <1000/mm3) and follow their WBC counts until recovery.
5.11 Seizures/Convulsions
In short-term, placebo-controlled trials, patients with a history of seizures excluded seizures/convulsions occurred in 0.1% (3/2467) of undiagnosed adult patients treated with oral aripiprazole, in 0.1% (1/732) of pediatric patients (6 to 18 years).
As with other antipsychotic drugs, aripiprazole should be used cautiously in patients with a history of seizures or with conditions that lower the seizure threshold. Conditions that lower the seizure threshold may be more prevalent in a population of 65 years or older.
5.12 Potential for Cognitive and Motor Impairment
Aripiprazole, like other antipsychotics, may have the potential to impair judgment, thinking, or motor skills. For example, in short-term, placebo-controlled trials, somnolence (including sedation) was reported as follows (aripiprazole incidence, placebo incidence): in adult patients (n=2467) treated with oral aripiprazole (11%, 6%), in pediatric patients ages 6 to 17 (n=611) (24%, 6%). Somnolence (including sedation) led to discontinuation in 0.3% (8/2467) of adult patients and 3% (20/732) of pediatric patients (6 to 18 years) on oral aripiprazole in short-term, placebo-controlled trials.
Despite the relatively modest increased incidence of these events compared to placebo, patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that therapy with aripiprazole does not affect them adversely.
5.13 Body Temperature Regulation
Disruption of the body's ability to reduce core body temperature has been attributed to antipsychotic agents. Appropriate care is advised when prescribing aripiprazole for patients who will be experiencing conditions which may contribute to an elevation in core body temperature, (e.g., exercising strenuously, exposure to extreme heat, receiving concomitant medication with anticholinergic activity, or being subject to dehydration) [see Adverse Reactions (6.2)].
5.14 Suicide
The possibility of a suicide attempt is inherent in psychotic illnesses, bipolar disorder, and close supervision of high-risk patients should accompany drug therapy. Prescriptions for aripiprazole should be written for the smallest quantity consistent with good patient management in order to reduce the risk of overdose [see Adverse Reactions (6.1, 6.2)].
5.15 Dysphagia
Esophageal dysmotility and aspiration have been associated with antipsychotic drug use, including aripiprazole. Aspiration pneumonia is a common cause of morbidity and mortality in elderly patients, in particular those with advanced Alzheimer's dementia. Aripiprazole and other antipsychotic drugs should be used cautiously in patients at risk for aspiration pneumonia [see Warnings and Precautions (5.1) and Adverse Reactions (6.2)].
Close5.16 Risks in Patients with Phenylketonuria
Phenylalanine is a component of aspartame. Each aripiprazole orally disintegrating tablet contains the following amounts: 10 mg - 1.12 mg phenylalanine and 15 mg - 1.68 mg phenylalanine.
-
6 ADVERSE REACTIONSBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials ...
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Increased Mortality in Elderly Patients with Dementia-Related Psychosis [see Boxed Warning and Warnings and Precautions (5.1)]
- Cerebrovascular Adverse Events, Including Stroke [see Warnings and Precautions (5.2)]
- Suicidal Thoughts and Behaviors in Children, Adolescents, and Young Adults [see Boxed Warning and Warnings and Precautions (5.3)]
- Neuroleptic Malignant Syndrome (NMS) [see Warnings and Precautions (5.4)]
- Tardive Dyskinesia [see Warnings and Precautions (5.5)]
- Metabolic Changes [see Warnings and Precautions (5.6)]
- Pathological Gambling and Other Compulsive Behaviors [see Warnings and Precautions (5.7)]
- Orthostatic Hypotension [see Warnings and Precautions (5.8)]
- Falls [see Warnings and Precautions (5.9)]
- Leukopenia, Neutropenia, and Agranulocytosis [see Warnings and Precautions (5.10)]
- Seizures/Convulsions [see Warnings and Precautions (5.11)]
- Potential for Cognitive and Motor Impairment [see Warnings and Precautions (5.12)]
- Body Temperature Regulation [see Warnings and Precautions (5.13)]
- Suicide [see Warnings and Precautions (5.14)]
- Dysphagia [see Warnings and Precautions (5.15)]
The most common adverse reactions in adult patients in clinical trials (≥10%) were nausea, vomiting, constipation, headache, dizziness, akathisia, anxiety, insomnia, and restlessness.
The most common adverse reactions in the pediatric clinical trials (≥10%) were somnolence, headache, vomiting, extrapyramidal disorder, fatigue, increased appetite, insomnia, nausea, nasopharyngitis, and weight increased.
Aripiprazole has been evaluated for safety in 13,543 adult patients who participated in multiple-dose, clinical trials in schizophrenia, other indications, Dementia of the Alzheimer's type, Parkinson's disease, and alcoholism, and who had approximately 7619 patient-years of exposure to oral aripiprazole and 749 patients with exposure to aripiprazole injection. A total of 3390 patients were treated with oral aripiprazole for at least 180 days and 1933 patients treated with oral aripiprazole had at least 1 year of exposure.
Aripiprazole has been evaluated for safety in 1,686 patients (6 to 18 years) who participated in multiple-dose, clinical trials in schizophrenia, autistic disorder, Tourette's disorder or other indications and who had approximately 1,342 patient-years of exposure to oral aripiprazole. A total of 959 pediatric patients were treated with oral aripiprazole for at least 180 days and 556 pediatric patients treated with oral aripiprazole had at least 1 year of exposure.
The conditions and duration of treatment with aripiprazole included (in overlapping categories) double-blind, comparative and noncomparative open-label studies, inpatient and outpatient studies, fixed- and flexible-dose studies, and short- and longer-term exposure.
6.1 Clinical Trials Experience
Adult Patients with Schizophrenia
The following findings are based on a pool of five placebo-controlled trials (four 4-week and one 6-week) in which oral aripiprazole was administered in doses ranging from 2 mg/day to 30 mg/day.
Commonly Observed Adverse Reactions
The only commonly observed adverse reaction associated with the use of aripiprazole in patients with schizophrenia (incidence of 5% or greater and aripiprazole incidence at least twice that for placebo) was akathisia (aripiprazole 8%; placebo 4%).
Less Common Adverse Reactions in Adults
Table 17 enumerates the pooled incidence, rounded to the nearest percent, of adverse reactions that occurred during acute therapy (up to 6 weeks in schizophrenia and up to 3 weeks in another indication), including only those reactions that occurred in 2% or more of patients treated with aripiprazole (doses ≥2 mg/day) and for which the incidence in patients treated with aripiprazole was greater than the incidence in patients treated with placebo in the combined dataset.
Table 17: Adverse Reactions in Short-Term, Placebo-Controlled Trials in Adult Patients Treated with Oral Aripiprazole - *
- Adverse reactions reported by at least 2% of patients treated with oral aripiprazole, except adverse reactions which had an incidence equal to or less than placebo.
Percentage of Patients Reporting Reaction*
System Organ Class
Preferred Term
Aripiprazole
(n=1843)
Placebo
(n=1166)
Eye Disorders
Blurred Vision
3
1
Gastrointestinal Disorders
Nausea
15
11
Constipation
11
7
Vomiting
11
6
Dyspepsia
9
7
Dry Mouth
5
4
Toothache
4
3
Abdominal Discomfort
3
2
Stomach Discomfort
3
2
General Disorders and Administration Site Conditions
Fatigue
6
4
Pain
3
2
Musculoskeletal and Connective Tissue Disorders
Musculoskeletal Stiffness
4
3
Pain in Extremity
4
2
Myalgia
2
1
Muscle Spasms
2
1
Nervous System Disorders
Headache
27
23
Dizziness
10
7
Akathisia
10
4
Sedation
7
4
Extrapyramidal Disorder
5
3
Tremor
5
3
Somnolence
5
3
Psychiatric Disorders
Agitation
19
17
Insomnia
18
13
Anxiety
17
13
Restlessness
5
3
Respiratory, Thoracic, and Mediastinal Disorders
Pharyngolaryngeal Pain
3
2
Cough
3
2
An examination of population subgroups did not reveal any clear evidence of differential adverse reaction incidence on the basis of age, gender, or race.
Pediatric Patients (13 to 17 years) with Schizophrenia
The following findings are based on one 6-week, placebo-controlled trial in which oral aripiprazole was administered in doses ranging from 2 to 30 mg/day.
Adverse Reactions Associated with Discontinuation of Treatment
The incidence of discontinuation due to adverse reactions between aripiprazole-treated and placebo-treated pediatric patients (13 to 17 years) was 5% and 2%, respectively.
Commonly Observed Adverse Reactions
Commonly observed adverse reactions associated with the use of aripiprazole in adolescent patients with schizophrenia (incidence of 5% or greater and aripiprazole incidence at least twice that for placebo) were extrapyramidal disorder, somnolence, and tremor.
Pediatric Patients (6 to 17 years) with Autistic Disorder
The following findings are based on two 8-week, placebo-controlled trials in which oral aripiprazole tablet was administered in doses of 2 to 15 mg/day.
Adverse Reactions Associated with Discontinuation of Treatment
The incidence of discontinuation due to adverse reactions between aripiprazole-treated and placebo-treated pediatric patients (6 to 17 years) was 10% and 8%, respectively.
Commonly Observed Adverse Reactions
Commonly observed adverse reactions associated with the use of aripiprazole tablet in pediatric patients with autistic disorder (incidence of 5% or greater and aripiprazole incidence at least twice that for placebo) are shown in Table 20.
Table 20: Commonly Observed Adverse Reactions in Short-Term, Placebo-Controlled Trials of Pediatric Patients (6 to 17 years) with Autistic Disorder Treated with Oral Aripiprazole Percentage of Patients Reporting Reaction
Preferred Term
Aripiprazole
(n=212)
Placebo
(n=101)
Sedation
21
4
Fatigue
17
2
Vomiting
14
7
Somnolence
10
4
Tremor
10
0
Pyrexia
9
1
Drooling
9
0
Decreased Appetite
7
2
Salivary Hypersecretion
6
1
Extrapyramidal Disorder
6
0
Lethargy
5
0
Pediatric Patients (6 to 18 years) with Tourette's Disorder
The following findings are based on one 8-week and one 10-week, placebo-controlled trials in which oral Aripiprazole was administered in doses of 2 to 20 mg/day.
Adverse Reactions Associated with Discontinuation of Treatment
The incidence of discontinuation due to adverse reactions between Aripiprazole-treated and placebo-treated pediatric patients (6 to 18 years) was 7% and 1%, respectively.
Commonly Observed Adverse Reactions
Commonly observed adverse reactions associated with the use of Aripiprazole in pediatric patients with Tourette's disorder (incidence of 5% or greater and Aripiprazole incidence at least twice that for placebo) are shown in Table 21.
Table 21: Commonly Observed Adverse Reactions in Short-Term, Placebo-Controlled Trials of Pediatric Patients (6 to 18 years) with Tourette's Disorder Treated with Oral Aripiprazole
Percentage of Patients Reporting Reaction
Preferred Term
Aripiprazole
(n=121)
Placebo
(n=72)
Sedation
13
6
Somnolence
13
1
Nausea
11
4
Headache
10
3
Nasopharyngitis
9
0
Fatigue
8
0
Increased Appetite
7
1
Less Common Adverse Reactions in Pediatric Patients (6 to 18 years) with Schizophrenia, Autistic Disorder, Tourette's Disorder or Other Indications.
Table 22 enumerates the pooled incidence, rounded to the nearest percent, of adverse reactions that occurred during acute therapy (up to 6 weeks in schizophrenia, up to 4 weeks in one indication, up to 8 weeks in autistic disorder, and up to 10-weeks in Tourette's disorder), including only those reactions that occurred in 2% or more of pediatric patients treated with aripiprazole (doses ≥2 mg/day) and for which the incidence in patients treated with aripiprazole was greater than the incidence in patients treated with placebo.
Table 22: Adverse Reactions in Short-Term, Placebo-Controlled Trials of Pediatric Patients (6 to 18 years) Treated with Oral Aripiprazole - *
- Adverse reactions reported by at least 2% of pediatric patients treated with oral aripiprazole, except adverse reactions which had an incidence equal to or less than placebo.
System Organ Class
Preferred Term
Percentage of Patients Reporting Reaction*
Aripiprazole
(n=732)
Placebo
(n=370)
Eye Disorders
Blurred Vision
3
0
Gastrointestinal Disorders
Abdominal Discomfort
2
1
Vomiting
8
7
Nausea
8
4
Diarrhea
4
3
Salivary Hypersecretion
4
1
Abdominal Pain Upper
3
2
Constipation
2
2
General Disorders and Administration Site Conditions
Fatigue
10
2
Pyrexia
4
1
Irritability
2
1
Asthenia
2
1
Infections and Infestations
Nasopharyngitis
6
3
Investigations
Weight Increased
3
1
Metabolism and Nutrition Disorders
Increased Appetite
7
3
Decreased Appetite
5
4
Musculoskeletal and Connective Tissue Disorders
Musculoskeletal Stiffness
2
1
Muscle Rigidity
2
1
Nervous System Disorders
Somnolence
16
4
Headache
12
10
Sedation
9
2
Tremor
9
1
Extrapyramidal Disorder
6
1
Akathisia
6
4
Drooling
3
0
Lethargy
3
0
Dizziness
3
2
Dystonia
2
1
Respiratory, Thoracic, and Mediastinal Disorders
Epistaxis
2
1
Skin and Subcutaneous Tissue Disorders
Rash
2
1
Dose-Related Adverse Reactions
Schizophrenia
Dose response relationships for the incidence of treatment-emergent adverse events were evaluated from four trials in adult patients with schizophrenia comparing various fixed doses (2, 5, 10, 15, 20, and 30 mg/day) of oral aripiprazole to placebo. This analysis, stratified by study, indicated that the only adverse reaction to have a possible dose response relationship, and then most prominent only with 30 mg, was somnolence [including sedation]; (incidences were placebo, 7.1%; 10 mg, 8.5%; 15 mg, 8.7%; 20 mg, 7.5%; 30 mg, 12.6%).
In the study of pediatric patients (13 to 17 years of age) with schizophrenia, three common adverse reactions appeared to have a possible dose response relationship: extrapyramidal disorder (incidences were placebo, 5.0%; 10 mg, 13%; 30 mg, 21.6%); somnolence (incidences were placebo, 6%; 10 mg, 11%; 30 mg, 21.6%); and tremor (incidences were placebo, 2%; 10 mg, 2%; 30 mg, 11.8%).
Autistic Disorder
In a study of pediatric patients (6 to 17 years of age) with autistic disorder, one common adverse reaction had a possible dose response relationship: fatigue (incidences were placebo, 0%; 5 mg, 3.8%; 10 mg, 22.0%; 15 mg, 18.5%).
Tourette's Disorder
In a study of pediatric patients (7 to 17 years of age) with Tourette's disorder, no common adverse reaction(s) had a dose response relationship.
Extrapyramidal Symptoms
Schizophrenia
In short-term, placebo-controlled trials in schizophrenia in adults, the incidence of reported EPS-related events, excluding events related to akathisia, for aripiprazole-treated patients was 13% vs. 12% for placebo; and the incidence of akathisia-related events for aripiprazole-treated patients was 8% vs. 4% for placebo. In the short-term, placebo-controlled trial of schizophrenia in pediatric patients (13 to 17 years), the incidence of reported EPS-related events, excluding events related to akathisia, for aripiprazole-treated patients was 25% vs. 7% for placebo; and the incidence of akathisia-related events for aripiprazole-treated patients was 9% vs. 6% for placebo.
Objectively collected data from those trials was collected on the Simpson Angus Rating Scale (for EPS), the Barnes Akathisia Scale (for akathisia), and the Assessments of Involuntary Movement Scales (for dyskinesias). In the adult schizophrenia trials, the objectively collected data did not show a difference between aripiprazole and placebo, with the exception of the Barnes Akathisia Scale (aripiprazole, 0.08; placebo, –0.05). In the pediatric (13 to 17 years) schizophrenia trial, the objectively collected data did not show a difference between aripiprazole and placebo, with the exception of the Simpson Angus Rating Scale (aripiprazole, 0.24; placebo, –0.29).
Similarly, in a long-term (26-week), placebo-controlled trial of schizophrenia in adults, objectively collected data on the Simpson Angus Rating Scale (for EPS), the Barnes Akathisia Scale (for akathisia), and the Assessments of Involuntary Movement Scales (for dyskinesias) did not show a difference between aripiprazole and placebo.
Autistic Disorder
In the short-term, placebo-controlled trials in autistic disorder in pediatric patients (6 to 17 years), the incidence of reported EPS-related events, excluding events related to akathisia, for aripiprazole-treated patients was 18% vs. 2% for placebo and the incidence of akathisia-related events for aripiprazole-treated patients was 3% vs. 9% for placebo.
In the pediatric (6 to 17 years) short-term autistic disorder trials, the Simpson Angus Rating Scale showed a significant difference between aripiprazole and placebo (aripiprazole, 0.1; placebo, – 0.4). Changes in the Barnes Akathisia Scale and the Assessments of Involuntary Movement Scales were similar for the aripiprazole and placebo groups.
Tourette's Disorder
In the short-term, placebo-controlled trials in Tourette's disorder in pediatric patients (6 to 18 years), the incidence of reported EPS-related events, excluding events related to akathisia, for Aripiprazole-treated patients was 7% vs. 6% for placebo and the incidence of akathisia-related events for Aripiprazole-treated patients was 4% vs. 6% for placebo.
In the pediatric (6 to 18 years) short-term Tourette's disorder trials, changes in the Simpson Angus Rating Scale, Barnes Akathisia Scale and Assessments of Involuntary Movement Scale were not clinically meaningfully different for Aripiprazole and placebo.
Dystonia
Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups.
Additional Findings Observed in Clinical Trials
Adverse Reactions in Long-Term, Double-Blind, Placebo-Controlled Trials
The adverse reactions reported in a 26-week, double-blind trial comparing oral aripiprazole and placebo in patients with schizophrenia were generally consistent with those reported in the short-term, placebo-controlled trials, except for a higher incidence of tremor [8% (12/153) for aripiprazole vs. 2% (3/153) for placebo]. In this study, the majority of the cases of tremor were of mild intensity (8/12 mild and 4/12 moderate), occurred early in therapy (9/12 ≤49 days), and were of limited duration (7/12 ≤10 days). Tremor infrequently led to discontinuation (<1%) of aripiprazole. In addition, in a long-term (52 week), active-controlled study, the incidence of tremor was 5% (40/859) for aripiprazole. A similar profile was observed in a long-term monotherapy study and a long-term adjunctive study with lithium and valproate in bipolar disorder.
Other Adverse Reactions Observed During Clinical Trial Evaluation of Aripiprazole
The following listing does not include reactions: 1) already listed in previous tables or elsewhere in labeling, 2) for which a drug cause was remote, 3) which were so general as to be uninformative, 4) which were not considered to have significant clinical implications, or 5) which occurred at a rate equal to or less than placebo.
Reactions are categorized by body system according to the following definitions: frequent adverse reactions are those occurring in at least 1/100 patients; infrequent adverse reactions are those occurring in 1/100 to 1/1000 patients; rare reactions are those occurring in fewer than 1/1000 patients:
Adults - Oral Administration
Blood and Lymphatic System Disorders:
rare - thrombocytopenia
Cardiac Disorders
infrequent – bradycardia, palpitations,
rare – atrial flutter, cardio-respiratory arrest, atrioventricular block, atrial fibrillation, angina pectoris, myocardial ischemia, myocardial infarction, cardiopulmonary failure
Eye Disorders
infrequent – photophobia; rare -diplopia
Gastrointestinal Disorders
infrequent - gastroesophageal reflux disease
General Disorders and Administration Site Conditions
frequent - asthenia; infrequent – peripheral edema, chest pain; rare – face edema
Hepatobiliary Disorders
rare - hepatitis, jaundice
Immune System Disorders
rare-hypersensitivity
Injury, Poisoning, and Procedural Complications
infrequent– fall; rare – heat stroke
Investigations
frequent - blood prolactin decreased, weight decreased, infrequent - hepatic enzyme increased, blood glucose increased, blood lactate dehydrogenase increased, gamma glutamyl transferase increased; rare – blood prolactin increased, blood urea increased, blood creatinine increased, blood bilirubin increased, electrocardiogram QT prolonged, glycosylated hemoglobin increased
Metabolism and Nutrition Disorders
frequent –anorexia; rare -hypokalemia, hyponatremia, hypoglycemia
Musculoskeletal and Connective Tissue Disorders
infrequent -muscular weakness, muscle tightness; rare – rhabdomyolysis, mobility decreased
Nervous System Disorders
infrequent - parkinsonism, memory impairment, cogwheel rigidity, hypokinesia, bradykinesia; rare – akinesia, myoclonus, coordination abnormal, speech disorder, Grand Mal convulsion; <1/10,000 patients -choreoathetosis
Psychiatric Disorders
infrequent – aggression, loss of libido, delirium; rare – libido increased, anorgasmia, tic, homicidal ideation, catatonia, sleep walking
Renal and Urinary Disorders
rare - urinary retention, nocturia
Reproductive System and Breast Disorders
infrequent - erectile dysfunction; rare – gynaecomastia, menstruation irregular, amenorrhea, breast pain, priapism
Respiratory, Thoracic, and Mediastinal Disorders
infrequent -nasal congestion, dyspnea
Skin and Subcutaneous Tissue Disorders
infrequent - rash, hyperhidrosis, pruritus, photosensitivity reaction, alopecia; rare- urticaria
Vascular Disorders
infrequent – hypotension, hypertension
Pediatric Patients - Oral Administration
Most adverse events observed in the pooled database of 1,686 pediatric patients, aged 6 to 18 years, were also observed in the adult population. Additional adverse reactions observed in the pediatric population are listed below.
Eye Disorders
infrequent - oculogyric crisis
Gastrointestinal Disorders
infrequent -tongue dry, tongue spasm
Investigations
frequent -blood insulin increased
Nervous System Disorders
infrequent - sleep talking
Renal and Urinary Disorders
frequent – enuresis
Skin and Subcutaneous Tissue Disorders
infrequent - hirsutism
Close6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of aripiprazole. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to establish a causal relationship to drug exposure: occurrences of allergic reaction (anaphylactic reaction, angioedema, laryngospasm, pruritus/urticaria, or oropharyngeal spasm), pathological gambling, hiccups, blood glucose fluctuation, oculogyric crisis, drug reaction with eosinophilia and systemic symptoms (DRESS) and fecal incontinence.
-
7 DRUG INTERACTIONS7.1 Drugs Having Clinically Important Interactions with Aripiprazole - Table 25: Clinically Important Drug Interactions with Aripiprazole - Concomitant Drug Name or Drug Class ...
7.1 Drugs Having Clinically Important Interactions with Aripiprazole
Table 25: Clinically Important Drug Interactions with Aripiprazole Concomitant Drug Name or Drug Class
Clinical Rationale
Clinical Recommendation
Strong CYP3A4 Inhibitors (e.g., itraconazole, clarithromycin) or strong CYP2D6 inhibitors (e.g., quinidine, fluoxetine, paroxetine)
The concomitant use of aripiprazole with strong CYP 3A4 or CYP2D6 inhibitors increased the exposure of aripiprazole compared to the use of aripiprazole alone [see Clinical Pharmacology (12.3)].
With concomitant use of aripiprazole with a strong CYP3A4 inhibitor or CYP2D6 inhibitor, reduce the aripiprazole dosage [see Dosage and Administration (2.7)].
Strong CYP3A4 Inducers (e.g., carbamazepine, rifampin)
The concomitant use of aripiprazole and carbamazepine decreased the exposure of aripiprazole compared to the use of aripiprazole alone [see Clinical Pharmacology (12.3)].
With concomitant use of aripiprazole with a strong CYP3A4 inducer, consider increasing the aripiprazole dosage [see Dosage and Administration (2.7)].
Antihypertensive Drugs
Due to its alpha adrenergic antagonism, aripiprazole has the potential to enhance the effect of certain antihypertensive agents.
Monitor blood pressure and adjust dose accordingly [see Warnings and Precautions (5.8)].
Benzodiazepines (e.g., lorazepam)
The intensity of sedation was greater with the combination of oral aripiprazole and lorazepam as compared to that observed with aripiprazole alone. The orthostatic hypotension observed was greater with the combination as compared to that observed with lorazepam alone [see Warnings and Precautions (5.8)].
Monitor sedation and blood pressure. Adjust dose accordingly.
Close7.2 Drugs Having No Clinically Important Interactions with Aripiprazole
Based on pharmacokinetic studies, no dosage adjustment of aripiprazole is required when administered concomitantly with famotidine, valproate, lithium, lorazepam.
In addition, no dosage adjustment is necessary for substrates of CYP2D6 (e.g., dextromethorphan, fluoxetine, paroxetine, or venlafaxine), CYP2C9 (e.g., warfarin), CYP2C19 (e.g., omeprazole, warfarin, escitalopram), or CYP3A4 (e.g., dextromethorphan) when co-administered with aripiprazole. Additionally, no dosage adjustment is necessary for valproate, lithium, lamotrigine, lorazepam, or sertraline when co-administered with aripiprazole. [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Pregnancy Exposure Registry - There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to atypical antipsychotics, including aripiprazole, during ...
8.1 Pregnancy
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to atypical antipsychotics, including aripiprazole, during pregnancy. Healthcare providers are encouraged to register patients by contacting the National Pregnancy Registry for Atypical Antipsychotics at 1-866-961-2388 or visit http://womensmentalhealth.org/clinical-and-researchprograms/pregnancyregistry/.
Risk Summary
Neonates exposed to antipsychotic drugs, including aripiprazole, during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery (see Clinical Considerations). Overall available data from published epidemiologic studies of pregnant women exposed to aripiprazole have not established a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes (see Data). There are risks to the mother associated with untreated schizophrenia, bipolar I disorder, or another indication, and with exposure to antipsychotics, including aripiprazole, during pregnancy (see Clinical Considerations). Aripiprazole exposure during pregnancy can have variable effects on milk supply in the post-partum period. [see Use in Specific Populations (8.2)].
In animal reproduction studies, oral and intravenous aripiprazole administration during organogenesis in rats and/or rabbits at doses 10 and 19 times, respectively, the maximum recommended human dose (MRHD) of 30 mg/day based on mg/m2 body surface area, produced fetal death, decreased fetal weight, undescended testicles, delayed skeletal ossification, skeletal abnormalities, and diaphragmatic hernia. Oral and intravenous aripiprazole administration during the pre- and post-natal period in rats at doses 10 times the MRHD based on mg/m2 body surface area, produced prolonged gestation, stillbirths, decreased pup weight, and decreased pup survival (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
There is a risk to the mother from untreated schizophrenia or bipolar I disorder, including increased risk of relapse, hospitalization, and suicide. Schizophrenia and bipolar I disorder are associated with increased adverse perinatal outcomes, including preterm birth. It is not known if this is a direct result of the illness or other comorbid factors.
Fetal/Neonatal Adverse Reactions
Extrapyramidal and/or withdrawal symptoms, including agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and feeding disorder have been reported in neonates who were exposed to antipsychotic drugs (including aripiprazole) during the third trimester of pregnancy. These symptoms have varied in severity. Monitor neonates for extrapyramidal and/or withdrawal symptoms, and manage symptoms appropriately. Some neonates recovered within hours or days without specific treatment; others required prolonged hospitalization.
Data
Human Data
Published data from observational studies, birth registries, and case reports on the use of atypical antipsychotics during pregnancy do not report a clear association with antipsychotics and major birth defects. A retrospective study from a Medicaid database of 9258 women exposed to antipsychotics during pregnancy did not indicate an overall increased risk for major birth defects.
Animal Data
In animal studies, aripiprazole demonstrated developmental toxicity, including possible teratogenic effects in rats and rabbits.
In pregnant rats treated orally with aripiprazole during organogenesis at doses of 3, 10, and 30 mg/kg/day, which are approximately 1, 3 and 10 times the MRHD of 30 mg/day based on mg/m2 body surface area, a slight prolongation of gestation and delay in fetal development, as evidenced by decreased fetal weight and undescended testes, were observed at 10 times the MRHD. Delayed skeletal ossification was observed at 3 and 10 times the MRHD. Delivered offspring had increased incidences of hepatodiaphragmatic nodules and diaphragmatic hernia were observed at 10 times the MRHD (the other dose groups were not examined for these findings). Postnatally, delayed vaginal opening was seen at 3 and 10 times the MRHD. Impaired reproductive performance (decreased fertility rate, corpora lutea, implants, live fetuses, and increased post-implantation loss, likely mediated through effects on female offspring) were observed at 10 times the MRHD; however, there was no evidence to suggest that these developmental effects were secondary to maternal toxicity.
In pregnant rats injected intravenously with aripiprazole during organogenesis at doses of 3, 9, and 27 mg/kg/day, which are 1, 3, and 9 times the MRHD of 30 mg/day based on mg/m2 body surface area, decreased fetal weight and delayed skeletal ossification were observed at 9 times the MRHD; this dose also caused maternal toxicity.
In pregnant rabbits treated orally with aripiprazole during organogenesis at doses of 10, 30, and 100 mg/kg/day which are 6, 19, and 65 times the MRHD of 30 mg/day based on mg/m2 body surface area, decreased maternal food consumption, and increased abortions as well as increased fetal mortality were observed at 65 times the MRHD. Decreased fetal weight and increased incidence of fused sternebrae were observed at 19 and 65 times the MRHD.
In pregnant rabbits injected intravenously with aripiprazole during organogenesis at doses of 3, 10, and 30 mg/kg/day, which are 2, 6, and 19 times the MRHD of 30 mg/day based on mg/m2 body surface area, decreased fetal weight, increased fetal abnormalities (primarily skeletal), and decreased fetal skeletal ossification were observed at 19 times the MRHD; this dose also caused maternal toxicity. The fetal no-effect dose was 10 mg/kg/day, which is 6 times the MRHD.
In rats treated orally with aripiprazole peri- and post-natally from gestation day 17 through postpartum day 21 at doses of 3, 10, and 30 mg/kg/day which are 1, 3, and 10 times the MRHD of 30 mg/day based on mg/m2 body surface area slight maternal toxicity and slightly prolonged gestation were observed at 10 times the MRHD. An increase in stillbirths and, decreases in pup weight (persisting into adulthood) and survival were also seen at this dose.
In rats injected intravenously with aripiprazole from gestation day 6 through lactation day 20 at doses of 3, 8, and 20 mg/kg/day, which are 1, 3, and 6 times the MRHD of 30 mg/day based on mg/m2 body surface area, increased stillbirths were observed at 3 and 6 times the MRHD; and decreases in early postnatal pup weight and survival were observed at 6 times the MRHD; these doses also caused some maternal toxicity. There were no effects on postnatal behavioral and reproductive development.
8.2 Lactation
Aripiprazole is present in human breast milk. Based on published case reports and pharmacovigilance reports, aripiprazole exposure during pregnancy and/or the postpartum period can lead to variable effects on milk supply in the post-partum period, including clinically relevant decreases in milk supply which may be reversible with discontinuation of the drug There are also reports of aripiprazole exposure during pregnancy and no maternal milk supply in the post-partum period. Effects on milk supply are likely mediated through decreases in prolactin levels, which have been observed [see Adverse Reactions (6.2)]. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for aripiprazole, and any potential adverse effects on the breastfed infant from aripiprazole, or from the underlying maternal condition.
Clinical Considerations
Monitor infants exposed to aripiprazole through breastmilk for dehydration and lack of appropriate weight gain.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients with agitation associated with schizophrenia or bipolar mania have not been established.
The pharmacokinetics of aripiprazole and dehydro-aripiprazole in pediatric patients, 10 to 17 years of age, were similar to those in adults after correcting for the differences in body weight [see Clinical Pharmacology (12.3)].
Schizophrenia
Safety and effectiveness in pediatric patients with schizophrenia were established in a 6-week, placebo-controlled clinical trial in 202 pediatric patients aged 13 to 17 years [see Dosage and Administration (2.1), Adverse Reactions (6.1), and Clinical Studies (14.1)]. Although maintenance efficacy in pediatric patients has not been systematically evaluated, maintenance efficacy can be extrapolated from adult data along with comparisons of aripiprazole pharmacokinetic parameters in adult and pediatric patients.
Bipolar I Disorder
Although maintenance efficacy in pediatric patients has not been systematically evaluated, maintenance efficacy can be extrapolated from adult data along with comparisons of aripiprazole pharmacokinetic parameters in adult and pediatric patients.
The efficacy of adjunctive aripiprazole with concomitant lithium or valproate in the treatment of manic or mixed episodes in pediatric patients has not been systematically evaluated. However, such efficacy and lack of pharmacokinetic interaction between aripiprazole and lithium or valproate can be extrapolated from adult data, along with comparisons of aripiprazole pharmacokinetic parameters in adult and pediatric patients.
Irritability Associated with Autistic Disorder
Safety and effectiveness in pediatric patients demonstrating irritability associated with autistic disorder were established in two 8-week, placebo-controlled clinical trials in 212 pediatric patients aged 6 to 17 years [see Indications and Usage (1), Dosage and Administration (2.4), Adverse Reactions (6.1), and Clinical Studies (14.4)]. A maintenance trial was conducted in pediatric patients (6 to 17 years of age) with irritability associated with autistic disorder. The first phase of this trial was an open-label, flexibly dosed (aripiprazole 2 to 15 mg/day) phase in which patients were stabilized (defined as > 25% improvement on the ABC-I subscale, and a CGI-I rating of "much improved" or "very much improved") on aripiprazole for 12 consecutive weeks. Overall, 85 patients were stabilized and entered the second, 16-week, double-blind phase where they were randomized to either continue aripiprazole treatment or switch to placebo. In this trial, the efficacy of aripiprazole for the maintenance treatment of irritability associated with autistic disorder was not established.
Tourette's Disorder
Safety and effectiveness of aripiprazole in pediatric patients with Tourette's Disorder were established in one 8-week (aged 7 to 17) and one 10-week trial (aged 6 to 18) in 194 pediatric patients [see Dosage and Administration (2.5), Adverse Reactions (6.1), and Clinical Studies (14.5)]. Maintenance efficacy in pediatric patients has not been systematically evaluated.
Juvenile Animal Studies
Aripiprazole in juvenile rats caused mortality, CNS clinical signs, impaired memory and learning, and delayed sexual maturation when administered at oral doses of 10, 20, 40mg/kg/day from weaning (21 days old) through maturity (80 days old). At 40mg/kg/day, mortality, decreased activity, splayed hind limbs, hunched posture, ataxia, tremors and other CNS signs were observed in both genders. In addition, delayed sexual maturation was observed in males. At all doses and in a dose-dependent manner, impaired memory and learning, increased motor activity, and histopathology changes in the pituitary (atrophy), adrenals (adrenocortical hypertrophy), mammary glands (hyperplasia and increased secretion), and female reproductive organs (vaginal mucification, endometrial atrophy, decrease in ovarian corpora lutea) were observed. The changes in female reproductive organs were considered secondary to the increase in prolactin serum levels. A No Observed Adverse Effect Level (NOAEL) could not be determined and, at the lowest tested dose of 10mg/kg/day, there is no safety margin relative to the systemic exposures (AUC0-24) for aripiprazole or its major active metabolite in adolescents at the maximum recommended pediatric dose of 15 mg/day. All drug-related effects were reversible after a 2 month recovery period, and most of the drug effects in juvenile rats were also observed in adult rats from previously conducted studies.
Aripiprazole in juvenile dogs (2 months old) caused CNS clinical signs of tremors, hypoactivity, ataxia, recumbency and limited use of hind limbs when administered orally for 6 months at 3, 10, 30mg/kg/day. Mean body weight and weight gain were decreased up to 18% in females in all drug groups relative to control values. A NOAEL could not be determined and, at the lowest tested dose of 3mg/kg/day, there is no safety margin relative to the systemic exposures (AUC0-24) for aripiprazole or its major active metabolite in adolescents at the maximum recommended pediatric dose of 15 mg/day. All drug-related effects were reversible after a 2 month recovery period.
8.5 Geriatric Use
No dosage adjustment is recommended for elderly patients [see Boxed Warning, Warnings and Precautions (5.1), and Clinical Pharmacology (12.3)].
Of the 13,543 patients treated with oral aripiprazole in clinical trials, 1073 (8%) were ≥65 years old and 799 (6%) were ≥75 years old. Placebo-controlled studies of oral aripiprazole in schizophrenia, or other indications did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
Aripiprazole is not approved for the treatment of patients with psychosis associated with Alzheimer's disease [see Boxed Warning and Warnings and Precautions (5.1)].
8.6 CYP2D6 Poor Metabolizers
Dosage adjustment is recommended in known CYP2D6 poor metabolizers due to high aripiprazole concentrations. Approximately 8% of Caucasians and 3 - 8% of Black/African Americans cannot metabolize CYP2D6 substrates and are classified as poor metabolizers (PM) [see Dosage and Administration (2.7) and Clinical Pharmacology (12.3)].
8.7 Hepatic and Renal Impairment
No dosage adjustment for aripiprazole is required on the basis of a patient's hepatic function (mild to severe hepatic impairment, Child-Pugh score between 5 and 15), or renal function (mild to severe renal impairment, glomerular filtration rate between 15 and 90 mL/minute) [see Clinical Pharmacology (12.3)].
Close8.8 Other Specific Populations
No dosage adjustment for aripiprazole is required on the basis of a patient's sex, race, or smoking status [see Clinical Pharmacology (12.3)].
-
9 DRUG ABUSE AND DEPENDENCE9.1 Controlled Substance - Aripiprazole is not a controlled substance. 9.2 Abuse - Aripiprazole has not been systematically studied in humans for its potential for abuse, tolerance, or ...
9.2 Abuse
Aripiprazole has not been systematically studied in humans for its potential for abuse, tolerance, or physical dependence. Consequently, patients should be evaluated carefully for a history of drug abuse, and such patients should be observed closely for signs of aripiprazole misuse or abuse (e.g., development of tolerance, increases in dose, drug-seeking behavior).
Close9.3 Dependence
In physical dependence studies in monkeys, withdrawal symptoms were observed upon abrupt cessation of dosing. While the clinical trials did not reveal any tendency for any drug-seeking behavior, these observations were not systematic and it is not possible to predict on the basis of this limited experience the extent to which a CNS-active drug will be misused, diverted, and/or abused once marketed.
-
10 OVERDOSAGEMedDRA terminology has been used to classify the adverse reactions. 10.1 Human Experience - In clinical trials and in postmarketing experience, adverse reactions of deliberate or accidental ...
MedDRA terminology has been used to classify the adverse reactions.
10.1 Human Experience
In clinical trials and in postmarketing experience, adverse reactions of deliberate or accidental overdosage with oral aripiprazole have been reported worldwide. These include overdoses with oral aripiprazole alone and in combination with other substances. No fatality was reported with aripiprazole alone. The largest known dose with a known outcome involved acute ingestion of 1260 mg of oral aripiprazole (42 times the maximum recommended daily dose) by a patient who fully recovered. Deliberate or accidental overdosage was also reported in children (age 12 and younger) involving oral aripiprazole ingestions up to 195 mg with no fatalities.
Common adverse reactions (reported in at least 5% of all overdose cases) reported with oral aripiprazole overdosage (alone or in combination with other substances) include vomiting, somnolence, and tremor. Other clinically important signs and symptoms observed in one or more patients with aripiprazole overdoses (alone or with other substances) include acidosis, aggression, aspartate aminotransferase increased, atrial fibrillation, bradycardia, coma, confusional state, convulsion, blood creatine phosphokinase increased, depressed level of consciousness, hypertension, hypokalemia, hypotension, lethargy, loss of consciousness, QRS complex prolonged, QT prolonged, pneumonia aspiration, respiratory arrest, status epilepticus, and tachycardia.
Close10.2 Management of Overdosage
No specific information is available on the treatment of overdose with aripiprazole. An electrocardiogram should be obtained in case of overdosage and if QT interval prolongation is present, cardiac monitoring should be instituted. Otherwise, management of overdose should concentrate on supportive therapy, maintaining an adequate airway, oxygenation and ventilation, and management of symptoms. Close medical supervision and monitoring should continue until the patient recovers.
Charcoal: In the event of an overdose of aripiprazole, an early charcoal administration may be useful in partially preventing the absorption of aripiprazole. Administration of 50 g of activated charcoal, one hour after a single 15 mg oral dose of aripiprazole, decreased the mean AUC and Cmax of aripiprazole by 50%.
Hemodialysis: Although there is no information on the effect of hemodialysis in treating an overdose with aripiprazole, hemodialysis is unlikely to be useful in overdose management since aripiprazole is highly bound to plasma proteins.
-
11 DESCRIPTIONAripiprazole is an atypical antipsychotic drug that is available as aripiprazole orally disintegrating tablets. Aripiprazole is 7-[4-[4-(2,3-dichlorophenyl)-1 ...
Aripiprazole is an atypical antipsychotic drug that is available as aripiprazole orally disintegrating tablets. Aripiprazole is 7-[4-[4-(2,3-dichlorophenyl)-1- piperazinyl]butoxy]-3,4-dihydrocarbostyril. The molecular formula is C23H27Cl2N3O2 and its molecular weight is 448.39. The chemical structure is:
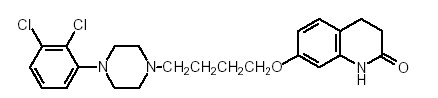
Each aripiprazole orally disintegrating tablet, USP intended for oral administration contains 10 mg or 15 mg of aripiprazole, USP. In addition, each tablet contains the following inactive ingredients: aspartame, calcium stearate, crospovidone, flavor firmenich powder peppermint and mannitol.
Close -
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - The mechanism of action of aripiprazole in schizophrenia or bipolar mania, is unclear. However, the efficacy of aripiprazole in the listed indications could be ...
12.1 Mechanism of Action
The mechanism of action of aripiprazole in schizophrenia or bipolar mania, is unclear. However, the efficacy of aripiprazole in the listed indications could be mediated through a combination of partial agonist activity at D2 and 5-HT1A receptors and antagonist activity at 5-HT2A receptors.
12.2 Pharmacodynamics
Aripiprazole exhibits high affinity for dopamine D2 and D3, serotonin 5-HT1A and 5-HT2A receptors (Ki values of 0.34 nM, 0.8 nM, 1.7 nM, and 3.4 nM, respectively), moderate affinity for dopamine D4, serotonin 5-HT2C and 5-HT7, alpha1-adrenergic and histamine H1 receptors (Ki values of 44 nM, 15 nM, 39 nM, 57 nM, and 61 nM, respectively), and moderate affinity for the serotonin reuptake site (Ki=98 nM). Aripiprazole has no appreciable affinity for cholinergic muscarinic receptors (IC50>1000 nM).
Close12.3 Pharmacokinetics
Aripiprazole activity is presumably primarily due to the parent drug, aripiprazole, and to a lesser extent, to its major metabolite, dehydro-aripiprazole, which has been shown to have affinities for D2 receptors similar to the parent drug and represents 40% of the parent drug exposure in plasma. The mean elimination half-lives are about 75 hours and 94 hours for aripiprazole and dehydro-aripiprazole, respectively. Steady-state concentrations are attained within 14 days of dosing for both active moieties.
Aripiprazole accumulation is predictable from single-dose pharmacokinetics. At steady-state, the pharmacokinetics of aripiprazole is dose-proportional. Elimination of aripiprazole is mainly through hepatic metabolism involving two P450 isozymes, CYP2D6 and CYP3A4. For CYP2D6 poor metabolizers, the mean elimination half-life for aripiprazole is about 146 hours.
Pharmacokinetic studies showed that aripiprazole orally disintegrating tablets are bioequivalent to aripiprazole tablets.
ORAL ADMINISTRATION
Absorption
Tablet: Aripiprazole is well absorbed after administration of the tablet, with peak plasma concentrations occurring within 3 hours to 5 hours; the absolute oral bioavailability of the tablet formulation is 87%. Aripiprazole can be administered with or without food. Administration of a 15 mg aripiprazole tablet with a standard high-fat meal did not significantly affect the Cmax or AUC of aripiprazole or its active metabolite, dehydro-aripiprazole, but delayed Tmax by 3 hours for aripiprazole and 12 hours for dehydro-aripiprazole.
Distribution
The steady-state volume of distribution of aripiprazole following intravenous administration is high (404 L or 4.9 L/kg), indicating extensive extravascular distribution. At therapeutic concentrations, aripiprazole and its major metabolite are greater than 99% bound to serum proteins, primarily to albumin. In healthy human volunteers administered 0.5 mg/day to 30 mg/day aripiprazole for 14 days, there was dose-dependent D2 receptor occupancy indicating brain penetration of aripiprazole in humans.
Metabolism and Elimination
Aripiprazole is metabolized primarily by three biotransformation pathways: dehydrogenation, hydroxylation, and N-dealkylation. Based on in vitro studies, CYP3A4 and CYP2D6 enzymes are responsible for dehydrogenation and hydroxylation of aripiprazole, and N-dealkylation is catalyzed by CYP3A4. Aripiprazole is the predominant drug moiety in the systemic circulation. At steady-state, dehydro-aripiprazole, the active metabolite, represents about 40% of aripiprazole AUC in plasma.
Following a single oral dose of [14C]-labeled aripiprazole, approximately 25% and 55% of the administered radioactivity was recovered in the urine and feces, respectively. Less than 1% of unchanged aripiprazole was excreted in the urine and approximately 18% of the oral dose was recovered unchanged in the feces.
Drug Interaction Studies
Effects of other drugs on the exposures of aripiprazole and dehydro-aripiprazole are summarized in Figure 1 and Figure 2, respectively. Based on simulation, a 4.5-fold increase in mean Cmax and AUC values at steady-state is expected when extensive metabolizers of CYP2D6 are administered with both strong CYP2D6 and CYP3A4 inhibitors. A 3-fold increase in mean Cmax and AUC values at steady-state is expected in poor metabolizers of CYP2D6 administered with strong CYP3A4 inhibitors.
Figure 1: The effects of other drugs on aripiprazole pharmacokinetics
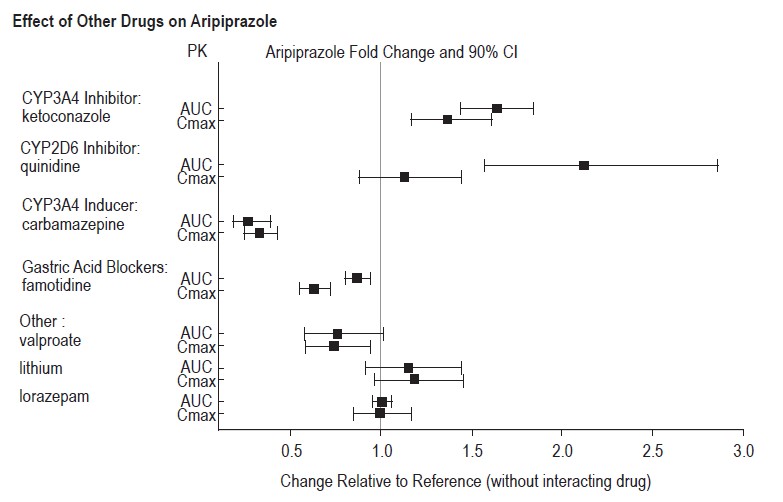
Figure 2: The effects of other drugs on dehydro-aripiprazole pharmacokinetics
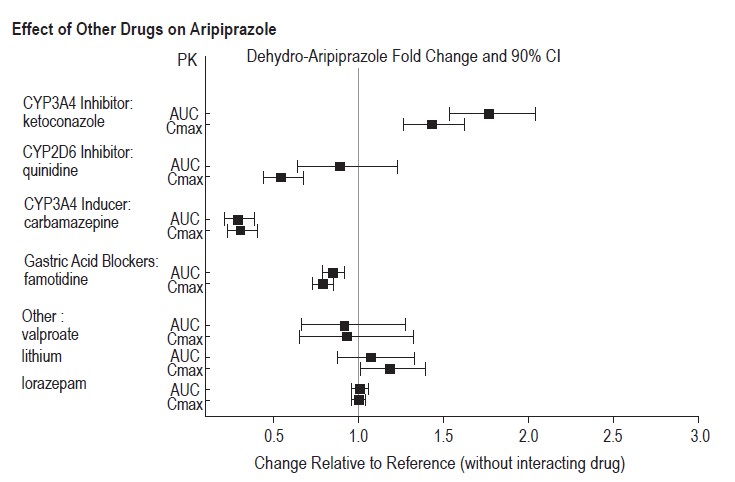
The effects of aripiprazole on the exposures of other drugs are summarized in Figure 3.
Figure 3:The effects of aripiprazole on pharmacokinetics of other drugs
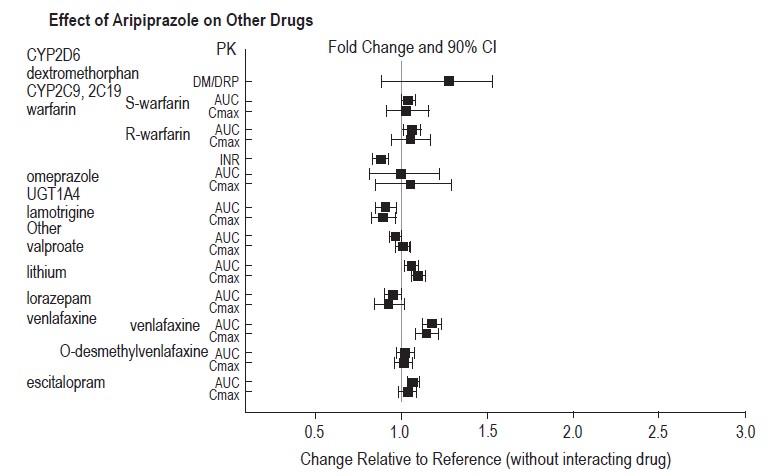
Studies in Specific Populations
Exposures of aripiprazole and dehydro-aripiprazole in specific populations are summarized in Figure 4 and Figure 5, respectively. In addition, in pediatric patients (10 to 17 years of age) administered with aripiprazole (20 mg to 30 mg), the body weight corrected aripiprazole clearance was similar to the adults.
Figure 4: Effects of intrinsic factors on aripiprazole pharmacokinetics
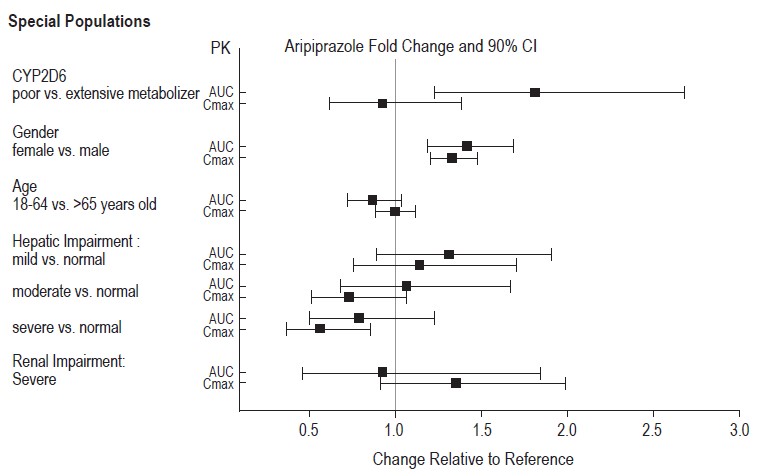
Figure 5: Effects of intrinsic factors on dehydro-aripiprazole pharmacokinetics
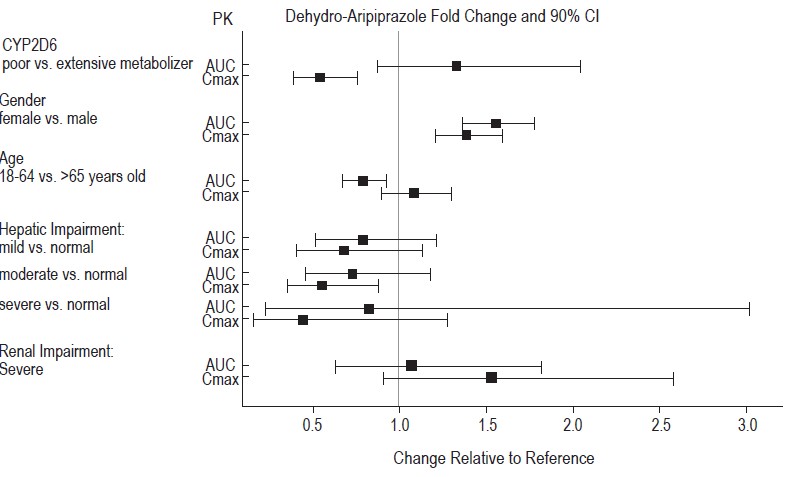
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Carcinogenesis - Lifetime carcinogenicity studies were conducted in ICR mice, F344 rats, and Sprague-Dawley (SD) rats. Aripiprazole ...
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Lifetime carcinogenicity studies were conducted in ICR mice, F344 rats, and Sprague-Dawley (SD) rats. Aripiprazole was administered for 2 years in the diet at doses of 1, 3, 10, and 30 mg/kg/day to ICR mice and 1, 3, and 10 mg/kg/day to F344 rats (0.2, 0.5, 2 and 5 times and 0.3, 1 and 3 times the MRHD of 30 mg/day based on mg/m2 body surface area, respectively). In addition, SD rats were dosed orally for 2 years at 10, 20, 40, and 60 mg/kg/day, which are 3, 6, 13 and 19 times the MRHD based on mg/m2 body surface area. Aripiprazole did not induce tumors in male mice or male rats. In female mice, the incidences of pituitary gland adenomas and mammary gland adenocarcinomas and adenoacanthomas were increased at dietary doses of 3 to 30 mg/kg/day (0.5 to 5 times the MRHD). In female rats, the incidence of mammary gland fibroadenomas was increased at a dietary dose of 10 mg/kg/day (3 times the MRHD); and the incidences of adrenocortical carcinomas and combined adrenocortical adenomas/carcinomas were increased at an oral dose of 60 mg/kg/day (19 times the MRHD).
An increase in mammary, pituitary, and endocrine pancreas neoplasms has been found in rodents after chronic administration of other antipsychotic drugs and is considered to be mediated by prolonged dopamine D2-receptor antagonism and hyperprolactinemia. Serum prolactin was not measured in the aripiprazole carcinogenicity studies. However, increases in serum prolactin levels were observed in female mice in a 13-week dietary study at the doses associated with mammary gland and pituitary tumors. Serum prolactin was not increased in female rats in 4-week and 13-week dietary studies at the dose associated with mammary gland tumors. The relevance for human risk of the findings of prolactin-mediated endocrine tumors in rodents is unclear.
Mutagenesis
The mutagenic potential of aripiprazole was tested in the in vitro bacterial reverse-mutation assay, the in vitro bacterial DNA repair assay, the in vitro forward gene mutation assay in mouse lymphoma cells, the in vitro chromosomal aberration assay in Chinese hamster lung (CHL) cells, the in vivo micronucleus assay in mice, and the unscheduled DNA synthesis assay in rats. Aripiprazole and a metabolite (2,3-DCPP) were clastogenic in the in vitro chromosomal aberration assay in CHL cells with and without metabolic activation. The metabolite, 2,3-DCPP, increased numerical aberrations in the in vitro assay in CHL cells in the absence of metabolic activation. A positive response was obtained in the in vivo micronucleus assay in mice; however, the response was due to a mechanism not considered relevant to humans.
Impairment of Fertility
Female rats were treated orally with aripiprazole from 2-weeks prior to mating through gestation day 7 at doses of 2, 6, and 20 mg/kg/day, which are 0.6, 2, and 6 times the MRHD of 30 mg/day based on mg/m2 body surface area. Estrus cycle irregularities and increased corpora lutea were seen at all doses, but no impairment of fertility was seen. Increased pre-implantation loss was seen at 2 and 6 times the MRHD, and decreased fetal weight was seen at 6 times the MRHD.
Male rats were treated orally with aripiprazole from 9-weeks prior to mating through mating at doses of 20, 40, and 60 mg/kg/day, which are 6, 13, and 19 times the MRHD of 30 mg/day based on mg/m2 body surface area. Disturbances in spermatogenesis were seen at 19 times the MRHD and prostate atrophy was seen at 13 and 19 times the MRHD without impairment of fertility.
Close13.2 Animal Toxicology and/or Pharmacology
Aripiprazole produced retinal degeneration in albino rats in a 26-week chronic toxicity study at a dose of 60 mg/kg/day and in a 2-year carcinogenicity study at doses of 40 and 60 mg/kg/day which are 13 and 19 times the MRHD of 30 mg/day based on mg/m2 body surface area. Evaluation of the retinas of albino mice and of monkeys did not reveal evidence of retinal degeneration. Additional studies to further evaluate the mechanism have not been performed. The relevance of this finding to human risk is unknown.
-
14 CLINICAL STUDIESEfficacy of the oral formulations of aripiprazole was established in the following adequate and well-controlled trials: Four short-term trials and one maintenance trial in adult patients and ...
Efficacy of the oral formulations of aripiprazole was established in the following adequate and well-controlled trials:
- Four short-term trials and one maintenance trial in adult patients and one short-term trial in adolescents (ages 13 to 17) with schizophrenia [see Clinical Studies (14.1)]
- One maintenance monotherapy trial and in one maintenance adjunctive trial in adult patients with bipolar I disorder [see Clinical Studies (14.2)]
- Two short-term trials in pediatric patients (ages 6 to 17 years) for the treatment of irritability associated with autistic disorder [see Clinical Studies (14.4)]
- Two short-term trials in pediatric patients (ages 6 to 18 years) with Tourette's disorder [see Clinical Studies (14.5)]
14.1 Schizophrenia
The efficacy of aripiprazole in the treatment of schizophrenia was evaluated in five short-term (4-week and 6-week), placebo-controlled trials of acutely relapsed inpatients who predominantly met DSM-III/IV criteria for schizophrenia. Four of the five trials were able to distinguish aripiprazole from placebo, but one study, the smallest, did not. Three of these studies also included an active control group consisting of either risperidone (one trial) or haloperidol (two trials), but they were not designed to allow for a comparison of aripiprazole and the active comparators.
In the four positive trials for aripiprazole, four primary measures were used for assessing psychiatric signs and symptoms. Efficacy was evaluated using the total score on the Positive and Negative Syndrome Scale (PANSS). The PANSS is a 30 item scale that measures positive symptoms of schizophrenia (7 items), negative symptoms of schizophrenia (7 items), and general psychopathology (16 items), each rated on a scale of 1 (absent) to 7 (extreme); total PANSS scores range from 30 to 210. The Clinical Global Impression (CGI) assessment reflects the impression of a skilled observer, fully familiar with the manifestations of schizophrenia, about the overall clinical state of the patient.
In a 4-week trial (n=414) comparing two fixed doses of aripiprazole (15 mg/day or 30 mg/day) to placebo, both doses of aripiprazole were superior to placebo in the PANSS total score (Study 1 in Table 26), PANSS positive subscale, and CGI-severity score. In addition, the 15 mg dose was superior to placebo in the PANSS negative subscale.
In a 4-week trial (n=404) comparing two fixed doses of aripiprazole (20 mg/day or 30 mg/day) to placebo, both doses of aripiprazole were superior to placebo in the PANSS total score (Study 2 in Table 26), PANSS positive subscale, PANSS negative subscale, and CGI-severity score.
In a 6-week trial (n=420) comparing three fixed doses of aripiprazole (10 mg/day, 15 mg/day, or 20 mg/day) to placebo, all three doses of aripiprazole were superior to placebo in the PANSS total score (Study 3 in Table 26), PANSS positive subscale, and the PANSS negative subscale.
In a 6-week trial (n=367) comparing three fixed doses of aripiprazole (2 mg/day, 5 mg/day, or 10 mg/day) to placebo, the 10 mg dose of aripiprazole was superior to placebo in the PANSS total score (Study 4 in Table 26), the primary outcome measure of the study. The 2 mg and 5 mg doses did not demonstrate superiority to placebo on the primary outcome measure.
Thus, the efficacy of 10 mg, 15 mg, 20 mg, and 30 mg daily doses was established in two studies for each dose. Among these doses, there was no evidence that the higher dose groups offered any advantage over the lowest dose group of these studies.
An examination of population subgroups did not reveal any clear evidence of differential responsiveness on the basis of age, gender, or race.
A longer-term trial enrolled 310 inpatients or outpatients meeting DSM-IV criteria for schizophrenia who were, by history, symptomatically stable on other antipsychotic medications for periods of 3 months or longer. These patients were discontinued from their antipsychotic medications and randomized to aripiprazole 15 mg/day or placebo for up to 26-weeks of observation for relapse. Relapse during the double-blind phase was defined as CGI-Improvement score of ≥5 (minimally worse), scores ≥5 (moderately severe) on the hostility or uncooperativeness items of the PANSS, or ≥20% increase in the PANSS total score. Patients receiving aripiprazole 15 mg/day experienced a significantly longer time to relapse over the subsequent 26-weeks compared to those receiving placebo (Study 5 in Figure 6).
Pediatric Patients
The efficacy of aripiprazole in the treatment of schizophrenia in pediatric patients (13 to 17 years of age) was evaluated in one 6-week, placebo-controlled trial of outpatients who met DSM-IV criteria for schizophrenia and had a PANSS score ≥70 at baseline. In this trial (n=302) comparing two fixed doses of aripiprazole (10 mg/day or 30 mg/day) to placebo, aripiprazole was titrated starting from 2 mg/day to the target dose in 5 days in the 10 mg/day treatment arm and in 11 days in the 30 mg/day treatment arm. Both doses of aripiprazole were superior to placebo in the PANSS total score (Study 6 in Table 26), the primary outcome measure of the study. The 30 mg/day dosage was not shown to be more efficacious than the 10 mg/day dose. Although maintenance efficacy in pediatric patients has not been systematically evaluated, maintenance efficacy can be extrapolated from adult data along with comparisons of aripiprazole pharmacokinetic parameters in adult and pediatric patients.
Table 26: Schizophrenia Studies Study Number
Treatment Group
Primary Efficacy Measure: PANSS
Mean Baseline Score (SD)
LS Mean Change from Baseline (SE)
Placebo-subtracted Difference* (95% CI)
Study 1
Aripiprazole
(15 mg/day) †
98.5 (17.2)
-15.5 (2.40)
-12.6 (-18.9, -6.2)
Aripiprazole
(30 mg/day)†
99.0 (19.2)
-11.4 (2.39)
-8.5 (-14.8, -2.1)
Placebo
100.2 (16.5)
-2.9 (2.36)
--
Study 2
Aripiprazole
(20 mg/day) †
92.6 (19.5)
-14.5 (2.23)
-9.6 (-15.4, -3.8)
Aripiprazole
(30 mg/day) †
94.2 (18.5)
-13.9 (2.24)
-9.0 (-14.8, -3.1)
Placebo
94.3 (18.5)
-5.0 (2.17)
--
Study 3
Aripiprazole
(10 mg/day) †
92.7 (19.5)
-15.0 (2.38)
-12.7 (-19, -6.41)
Aripiprazole
(15 mg/day) †
93.2 (21.6)
-11.7 (2.38)
-9.4 (-15.71, -3.08)
Aripiprazole
(20 mg/day) †
92.5 (20.9)
-14.4 (2.45)
-12.1 (-18.53, -5.68)
Placebo
92.3 (21.8)
-2.3 (2.35)
--
Study 4
Aripiprazole (2 mg/day)
90.7 (14.5)
-8.2 (1.90)
-2.9 (-8.29, 2.47)
Aripiprazole (5 mg/day)
92.0 (12.6)
-10.6 (1.93)
-5.2 (-10.7, 0.19)
Aripiprazole
(10 mg/day) †
90.0 (11.9)
-11.3 (1.88)
-5.9 (-11.3, -0.58)
Placebo
90.8 (13.3)
-5.3 (1.97)
--
Study 6 (Pediatric, 13 to 17 Years)
Aripiprazole
(10 mg/day) †
93.6 (15.7)
-26.7 (1.91)
-5.5 (-10.7, -0.21)
Aripiprazole
(30 mg/day)†
94.0 (16.1)
-28.6 (1.92)
-7.4 (-12.7, -2.13)
Placebo
94.6 (15.6)
-21.2 (1.93)
--
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval.
* Difference (drug minus placebo) in least-squares mean change from baseline.
†Doses statistically significantly superior to placebo.
Figure 6: Kaplan-Meier Estimation of Cumulative Proportion of Patients with Relapse (Schizophrenia Study 5)
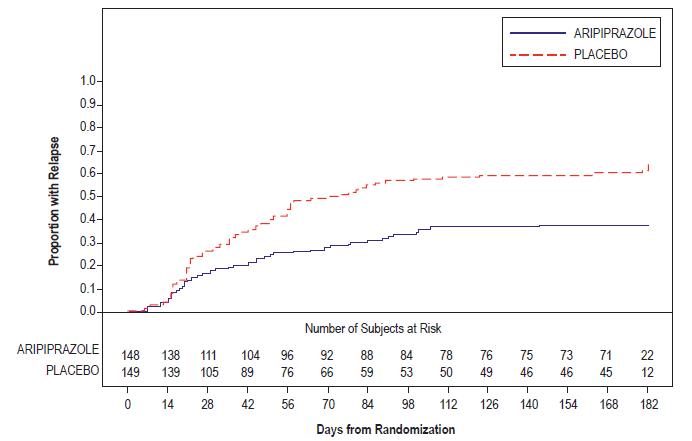
14.2 Bipolar Disorder
Maintenance Treatment of Bipolar I Disorder
Monotherapy Maintenance Therapy
A maintenance trial was conducted in adult patients meeting DSM-IV criteria for bipolar I disorder with a recent manic or mixed episode who had been stabilized on open-label aripiprazole and who had maintained a clinical response for at least 6-weeks. The first phase of this trial was an open-label stabilization period in which inpatients and outpatients were clinically stabilized and then maintained on open-label aripiprazole (15 mg/day or 30 mg/day, with a starting dose of 30 mg/day) for at least 6 consecutive weeks. One hundred sixty-one outpatients were then randomized in a double-blind fashion, to either the same dose of aripiprazole they were on at the end of the stabilization and maintenance period or placebo and were then monitored for manic or depressive relapse. During the randomization phase, aripiprazole was superior to placebo on time to the number of combined affective relapses (manic plus depressive), the primary outcome measure for this study (Study 7 in Figure 7). A total of 55 mood events were observed during the double-blind treatment phase. Nineteen were from the aripiprazole group and 36 were from the placebo group. The number of observed manic episodes in the aripiprazole group (6) were fewer than that in the placebo group (19), while the number of depressive episodes in the aripiprazole group (9) was similar to that in the placebo group (11).
An examination of population subgroups did not reveal any clear evidence of differential responsiveness on the basis of age and gender; however, there were insufficient numbers of patients in each of the ethnic groups to adequately assess inter-group differences.
Figure 7: Kaplan-Meier Estimation of Cumulative Proportion of Patients with Relapse (Bipolar Study 7)
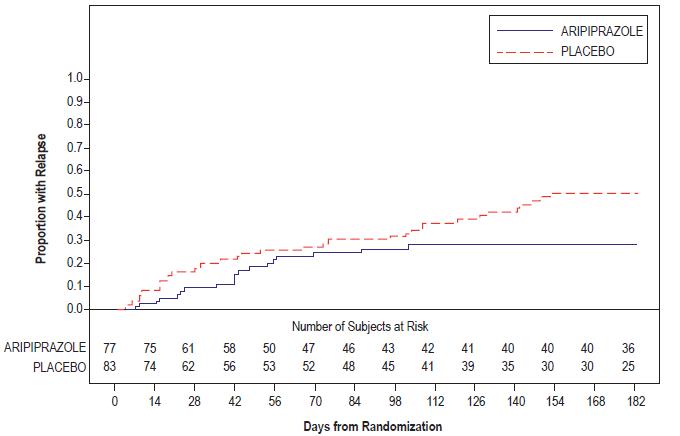
Adjunctive Maintenance Therapy
An adjunctive maintenance trial was conducted in adult patients meeting DSM-IV criteria for bipolar I disorder with a recent manic or mixed episode. Patients were initiated on open-label lithium (0.6 to 1.0 mEq/L) or valproate (50 to 125 mcg/mL) at therapeutic serum levels, and remained on stable doses for 2-weeks. At the end of 2-weeks, patients demonstrating inadequate response (Y-MRS total score ≥16 and ≤35% improvement on the Y-MRS total score) to lithium or valproate received aripiprazole with a starting dose of 15 mg/day with the option to increase to 30 mg or reduce to 10 mg as early as day 4, as adjunctive therapy with open-label lithium or valproate. Prior to randomization, patients on the combination of single-blind aripiprazole and lithium or valproate were required to maintain stability (Y-MRS and MADRS total scores ≤12) for 12 consecutive weeks. Three hundred thirty-seven patients were then randomized in a double-blind fashion, to either the same dose of aripiprazole they were on at the end of the stabilization period or placebo plus lithium or valproate and were then monitored for manic, mixed, or depressive relapse for a maximum of 52-weeks. Aripiprazole was superior to placebo on the primary endpoint, time from randomization to relapse to any mood event (Study 8 in Figure 8). A mood event was defined as hospitalization for a manic, mixed, or depressive episode, study discontinuation due to lack of efficacy accompanied by Y-MRS score >16 and/or a MADRS >16, or an SAE of worsening disease accompanied by Y-MRS score >16 and/or a MADRS >16. A total of 68 mood events were observed during the double-blind treatment phase. Twenty-five were from the aripiprazole group and 43 were from the placebo group. The number of observed manic episodes in the aripiprazole group (7) were fewer than that in the placebo group (19), while the number of depressive episodes in the aripiprazole group (14) was similar to that in the placebo group (18). The Kaplan-Meier curves of the time from randomization to relapse to any mood event during the 52-week, double-blind treatment phase for aripiprazole and placebo groups are shown in Figure 8.
Figure 8: Kaplan-Meier Estimation of Cumulative Proportion of Patients with Relapse to Any Mood Event (Bipolar Study 8)
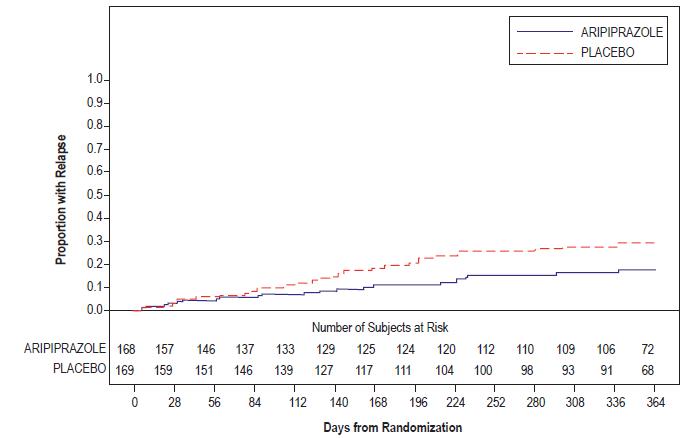
An examination of population subgroups did not reveal any clear evidence of differential responsiveness on the basis of age and gender; however, there were insufficient numbers of patients in each of the ethnic groups to adequately assess inter-group differences.
14.4 Irritability Associated with Autistic Disorder
The efficacy of aripiprazole in the treatment of irritability associated with autistic disorder was established in two 8-week, placebo-controlled trials in pediatric patients (6 to 17 years of age) who met the DSM-IV criteria for autistic disorder and demonstrated behaviors such as tantrums, aggression, self-injurious behavior, or a combination of these problems. Over 75% of these subjects were under 13 years of age.
Efficacy was evaluated using two assessment scales: the Aberrant Behavior Checklist (ABC) and the Clinical Global Impression-Improvement (CGI-I) scale. The primary outcome measure in both trials was the change from baseline to endpoint in the Irritability subscale of the ABC (ABC-I). The ABC-I subscale measured symptoms of irritability in autistic disorder.
The results of these trials are as follows:
In one of the 8-week, placebo-controlled trials, children and adolescents with autistic disorder (n=98), aged 6 to 17 years, received daily doses of placebo or aripiprazole 2 to 15 mg/day. aripiprazole, starting at 2 mg/day with increases allowed up to 15 mg/day based on clinical response, significantly improved scores on the ABC-I subscale and on the CGI-I scale compared with placebo. The mean daily dose of aripiprazole at the end of 8-week treatment was 8.6 mg/day (Study 1 in Table 29).
In the other 8-week, placebo-controlled trial in children and adolescents with autistic disorder (n=218), aged 6 to 17 years, three fixed doses of aripiprazole (5 mg/day, 10 mg/day, or 15 mg/day) were compared to placebo. aripiprazole dosing started at 2 mg/day and was increased to 5 mg/day after one week. After a second week, it was increased to 10 mg/day for patients in the 10 and 15 mg dose arms, and after a third week, it was increased to 15 mg/day in the 15 mg/day treatment arm (Study 2 in Table 29). All three doses of aripiprazole significantly improved scores on the ABC-I subscale compared with placebo.
Table 29: Irritability Associated with Autistic Disorder Studies (Pediatric) SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval.
* Difference (drug minus placebo) in least-squares mean change from baseline.
† Doses statistically significantly superior to placebo.
Study Number
Treatment Group
Primary Efficacy Measure: ABC-I
Mean Baseline Score (SD)
LS Mean Change from Baseline (SE)
Placebo-subtracted Difference* (95% CI)
Study 1
Aripiprazole (2-15 mg/day)†
29.6 (6.37)
-12.9 (1.44)
-7.9 (-11.7, -4.1)
Placebo
30.2 (6.52)
-5.0 (1.43)
--
Study 2
Aripiprazole (5 mg/day)†
28.6 (7.56)
-12.4 (1.36)
-4.0 (-7.7, -0.4)
Aripiprazole (10 mg/day)†
28.2 (7.36)
-13.2 (1.25)
-4.8 (-8.4, -1.3)
Aripiprazole (15 mg/day)†
28.9 (6.41)
-14.4 (1.31)
-6.0 (-9.6, -2.3)
Placebo
28.0 (6.89)
-8.4 (1.39)
--
Close14.5 Tourette's Disorder
The efficacy of aripiprazole in the treatment of Tourette's disorder was established in one 8-week (7 to 17 years of age) and one 10-week (6 to 18 years of age), placebo-controlled trials in pediatric patients (6 to 18 years of age) who met the DSM-IV criteria for Tourette's disorder and had a Total Tic score (TTS) ≥ 20 -22 on the Yale Global Tic Severity Scale (YGTSS). The YGTSS is a fully validated scale designed to measure current tic severity. Efficacy was evaluated using two assessment scales: 1) the Total Tic score (TTS) of the YGTSS and 2) the Clinical Global Impressions Scale for Tourette's Syndrome (CGI-TS), a clinician-determined summary measure that takes into account all available patient information. Over 65% of these patients were under 13 years of age.
The primary outcome measure in both trials was the change from baseline to endpoint in the TTS of the YGTSS. Ratings for the TTS are made along 5 different dimensions on a scale of 0 to 5 for motor and vocal tics each. Summation of these 10 scores provides a TTS (i.e., 0-50).
The results of these trials are as follows:
In the 8-week, placebo-controlled, fixed-dose trial, children and adolescents with Tourette's disorder (n=133), aged 7 to 17 years, were randomized 1:1:1 to low dose aripiprazole, high dose aripiprazole, or placebo. The target doses for the low and high dose aripiprazole groups were based on weight. Patients < 50 kg in the low dose aripiprazole group started at 2 mg per day with a target dose of 5 mg per day after 2 days. Patients ≥ 50 kg in the low dose aripiprazole group, started at 2 mg per day increased to 5 mg per day after 2 days, with a subsequent increase to a target dose of 10 mg per day at day 7. Patients <50 kg in the high dose aripiprazole group started at 2 mg per day increased to 5 mg per day after 2 days, with a subsequent increase to a target dose of 10 mg per day at day 7. Patients ≥ 50 kg in the high dose aripiprazole group, started at 2 mg per day increased to 5 mg per day after 2 days, with a subsequent increase to a dose of 10 mg per day at day 7 and were allowed weekly increases of 5 mg per day up to a target dose 20 mg per day at Day 21. Aripiprazole (both high and low dose groups) demonstrated statistically significantly improved scores on the YGTSS TTS (Study 1 in Table 30) and on the CGI-TS scale compared with placebo. The estimated improvements on the YGTSS TTS over the course of the study are displayed in Figure 9.
Figure 9: Least Square Means of Change from Baseline in YGTSS TTS by Week (Tourette's Disorder Study 1)
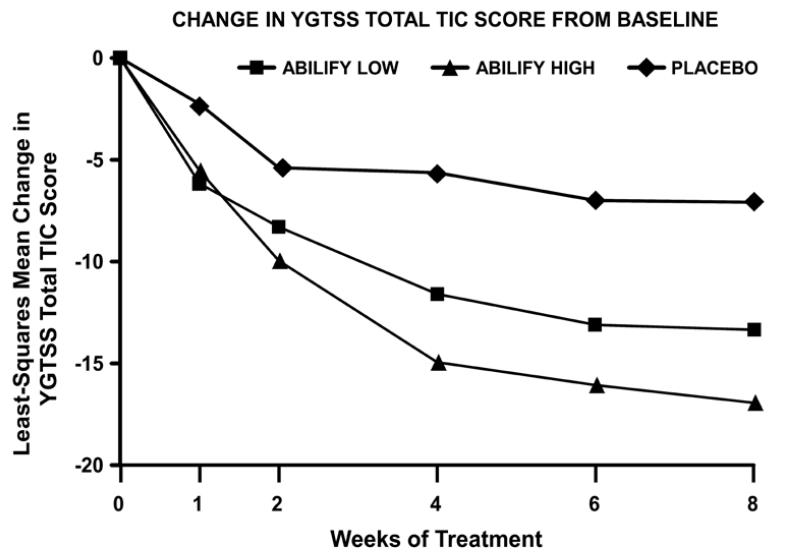
In the 10-week, placebo-controlled, flexible-dose trial in children and adolescents with Tourette's disorder (n=61), aged 6 to 18 years, patients received daily doses of placebo or aripiprazole, starting at 2 mg/day with increases allowed up to 20 mg/day based on clinical response. Aripiprazole demonstrated statistically significantly improved scores on the YGTSS TTS scale compared with placebo (Study 2 in Table 30). The mean daily dose of aripiprazole at the end of 10-week treatment was 6.54 mg/day.
Table 30: Tourette's Disorder Studies (Pediatric) Study
Treatment Group
Primary Efficacy Measure: YGTSS TTS
Number
Mean Baseline Score (SD)
LS Mean Change from Baseline (SE)
Placebo-subtracted Difference* (95% CI)
Study 1
Aripiprazole (low dose) †
29.2 (5.63)
-13.4 (1.59)
-6.3 (-10.2, -2.3)
Aripiprazole (high dose) †
31.2 (6.40)
-16.9 (1.61)
-9.9 (-13.8, -5.9)
Placebo
30.7 (5.95)
-7.1 (1.55)
--
Study 2
Aripiprazole (2-20 mg/day) †
28.3 (5.51)
-15.0 (1.51)
-5.3 (-9.8, -0.9)
Placebo
29.5 (5.60)
-9.6 (1.64)
--
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval.
* Difference (drug minus placebo) in least-squares mean change from baseline
† Doses statistically significantly superior to placebo
-
16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied - Aripiprazole Orally Disintegrating Tablets USP, 10 mg are white to off-white, capsule-shaped, uncoated tablets debossed with 'ZF 41' on one side and plain on the other side ...
16.1 How Supplied
Aripiprazole Orally Disintegrating Tablets USP, 10 mg are white to off-white, capsule-shaped, uncoated tablets debossed with 'ZF 41' on one side and plain on the other side and are supplied as follows:
NDC 72578-106-06 in bottle of 30 tablets
NDC 72578-106-78 in carton pack of 30 tablets (3x10's blister pack)
Aripiprazole Orally Disintegrating Tablets USP, 15 mg are white to off-white, round-shaped, biconvex, uncoated tablets debossed with 'ZF 42' on one side and plain on other side and are supplied as follows:
NDC 72578-107-06 in bottle of 30 tablets
NDC 72578-107-78 in carton pack of 30 tablets (3x10's blister pack)
Close16.2 Storage
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Dispense in a tight container.
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Medication Guide). Discuss the following issues with patients prescribed aripiprazole: Clinical Worsening of Depression and ...
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Discuss the following issues with patients prescribed aripiprazole:
Clinical Worsening of Depression and Suicide Risk
Patients, their families, and their caregivers should be encouraged to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, mania, other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during antidepressant treatment and when the dose is adjusted up or down. Families and caregivers of patients should be advised to look for the emergence of such symptoms on a day-to-day basis, since changes may be abrupt. Such symptoms should be reported to the patient's prescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patient's presenting symptoms. Symptoms such as these may be associated with an increased risk for suicidal thinking and behavior and indicate a need for very close monitoring and possibly changes in the medication [see Warnings and Precautions (5.3)].
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with aripiprazole and should counsel them in its appropriate use. A patient Medication Guide including information about "Antidepressant Medicines, Depression and other Serious Mental Illness, and Suicidal Thoughts or Actions" is available for aripiprazole. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. It should be noted that aripiprazole is not approved as a single agent for treatment of depression and has not been evaluated in pediatric major depressive disorder.
Pathological Gambling and Other Compulsive Behaviors
Advise patients and their caregivers of the possibility that they may experience compulsive urges to shop, intense urges to gamble, compulsive sexual urges, binge eating and/or other compulsive urges and the inability to control these urges while taking aripiprazole. In some cases, but not all, the urges were reported to have stopped when the dose was reduced or stopped [see Warnings and Precautions (5.7)].
Use of Orally Disintegrating Tablet
Do not open the bottle until ready to administer. Immediately upon opening the bottle, using dry hands, remove the tablet and place the entire aripiprazole orally disintegrating tablet on the tongue. After taking out the tablet immediately close the bottle.
Do not open the blister until ready to administer. For single tablet removal, open the package and peel back the foil on the blister to expose the tablet. Do not push the tablet through the foil because this could damage the tablet. Immediately upon opening the blister, using dry hands, remove the tablet and place the entire Aripiprazole Orally Disintegrating Tablet on the tongue.
Tablet disintegration occurs rapidly in saliva. It is recommended that aripiprazole orally disintegrating tablets be taken without liquid. However, if needed, it can be taken with liquid. Do not attempt to split the tablet.
Interference with Cognitive and Motor Performance
Because aripiprazole may have the potential to impair judgment, thinking, or motor skills, patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that aripiprazole therapy does not affect them adversely [see Warnings and Precautions (5.12)].
Concomitant Medication
Patients should be advised to inform their physicians if they are taking, or plan to take, any prescription or over-the-counter drugs, since there is a potential for interactions [see Drug Interactions (7)].
Heat Exposure and Dehydration
Patients should be advised regarding appropriate care in avoiding overheating and dehydration [see Warnings and Precautions (5.13)].
Pregnancy
Advise patients to notify their healthcare provider if they become pregnant or intend to become pregnant during treatment with aripiprazole. Advise patients that aripiprazole may cause extrapyramidal and/or withdrawal symptoms (agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, and feeding disorder) in a neonate. Advise patients that there is a pregnancy registry that monitors pregnancy outcomes in women exposed to aripiprazole during pregnancy [see Use in Specific Populations (8.1)].
Lactation
Aripiprazole use during pregnancy may affect milk supply. Advise the lactating patient to discuss any plans for breastfeeding with their healthcare provider, and to monitor the breastfed infant for dehydration and lack of appropriate weight gain [see Use in Specific Populations (8.2)].
Phenylketonurics
Phenylalanine is a component of aspartame. Each aripiprazole orally disintegrating tablet contains the following amounts: 10 mg - 1.12 mg phenylalanine and 15 mg - 1.68 mg phenylalanine.
Medication Guide available at www.vionausa.com/medguides or call 1-888-304-5011.
Manufactured by:
Square Pharmaceuticals Ltd.
Dhaka Unit, Kaliakoir, Gazipur-1750, Bangladesh
Distributed by:
Viona Pharmaceuticals Inc.
Cranford, NJ 07016
Rev.: 1/2025
Close -
MEDICATION GUIDEMEDICATION GUIDE - Aripiprazole (ar" i pip' ra zole) Orally Disintegrating Tablets, USP - What is the most important information I should know about aripiprazole orally disintegrating ...Close
MEDICATION GUIDE
Aripiprazole (ar" i pip' ra zole) Orally Disintegrating Tablets, USP
What is the most important information I should know about aripiprazole orally disintegrating tablets?
(For other side effects, also see " What are the possible side effects of aripiprazole orally disintegrating tablets ?")
Serious side effects may happen when you take aripiprazole orally disintegrating tablets, including:
- Increased risk of death in elderly patients with dementia-related psychosis: Medicines like aripiprazole orally disintegrating tablets can raise the risk of death in elderly people who have lost touch with reality (psychosis) due to confusion and memory loss (dementia). Aripiprazole orally disintegrating tablets are not approved for the treatment of patients with dementia-related psychosis.
- Risk of suicidal thoughts or actions : Antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions:
- Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
- Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions. These include people who have (or have a family history of) bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions.
- How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling very agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
What else do I need to know about antidepressant medicines?
- Never stop an antidepressant medicine without first talking to a healthcare provider . Stopping an antidepressant medicine suddenly can cause other symptoms.
- Antidepressants are medicines used to treat depression and other illnesses . It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
- Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
- Antidepressant medicines can interact with other medicines . Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
- Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child's healthcare provider for more information.
What are aripiprazole orally disintegrating tablets?
-
Aripiprazole orally disintegrating tablets are prescription medicines used to treat:
- Schizophrenia
- Irritability associated with autistic disorder
- Tourette's disorder
It is not known if aripiprazole orally disintegrating tablets are safe or effective in children:
- under 13 years of age with schizophrenia
- under 10 years of age with bipolar I disorder
- under 6 years of age with irritability associated with autistic disorder
- under 6 years of age with Tourette's disorder
Do not take aripiprazole orally disintegrating tablets if you are allergic to aripiprazole or any of the ingredients in aripiprazole orally disintegrating tablets. See the end of this Medication Guide for a complete list of ingredients in aripiprazole orally disintegrating tablets.
Before taking aripiprazole orally disintegrating tablets, tell your healthcare provider about all your medical conditions, including if you have or had:
- diabetes or high blood sugar in you or your family; your healthcare provider should check your blood sugar before you start aripiprazole orally disintegrating tablets and also during therapy.
- seizures (convulsions).
- low or high blood pressure.
- heart problems or stroke.
- pregnancy or plans to become pregnant. It is not known if aripiprazole orally disintegrating tablets will harm your unborn baby.
- If you become pregnant while receiving aripiprazole, talk to your healthcare provider about registering with the National Pregnancy Registry for Atypical Antipsychotics. You can register by calling 1-866-961-2388 or go to http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/.
- breast-feeding or plans to breast-feed. Aripiprazole passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you receive aripiprazole orally disintegrating tablets.
- low white blood cell count.
- phenylketonuria. Aripiprazole orally disintegrating tablets contain phenylalanine.
Tell your healthcare provider about all the medicines that you take , including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Aripiprazole orally disintegrating tablets and other medicines may affect each other causing possible serious side effects. Aripiprazole orally disintegrating tablets may affect the way other medicines work, and other medicines may affect how aripiprazole orally disintegrating tablets works.
Your healthcare provider can tell you if it is safe to take aripiprazole orally disintegrating tablets with your other medicines. Do not start or stop any medicines while taking aripiprazole orally disintegrating tablets without talking to your healthcare provider first. Know the medicines you take. Keep a list of your medicines to show your healthcare provider and pharmacist when you get a new medicine.
How should I take aripiprazole orally disintegrating tablets?
- Take aripiprazole orally disintegrating tablets exactly as your healthcare provider tells you to take it. Do not change the dose or stop taking aripiprazole orally disintegrating tablets yourself.
- Aripiprazole orally disintegrating tablets can be taken with or without food.
- If you miss a dose of aripiprazole orally disintegrating tablets, take the missed dose as soon as you remember. If it is almost time for the next dose, just skip the missed dose and take your next dose at the regular time. Do not take two doses of aripiprazole orally disintegrating tablets at the same time.
- If you have been prescribed aripiprazole orally disintegrating tablets, take it as follows:
- Do not open the blister until ready to take the orally disintegrating tablets.
- To remove one orally disintegrating tablet, open the package and peel back the foil on the blister to expose the tablet.
- Do not push the tablet through the foil because this could damage the tablet.
- Immediately upon opening the blister, using dry hands, remove the tablet and place the entire aripiprazole orally disintegrating tablet on the tongue.
- Do not open the bottle until ready to take the orally disintegrating tablets.
- Immediately upon opening the bottle, using dry hands, remove the tablet and place the entire aripiprazole orally disintegrating tablet on the tongue.
- Tablet disintegration occurs rapidly in saliva. It is recommended that aripiprazole orally disintegrating tablets be taken without liquid. However, if needed, it can be taken with liquid.
- Do not attempt to split the orally disintegrating tablet.
- If you take too much aripiprazole orally disintegrating tablets, call your healthcare provider or poison control center at 1-800-222-1222 right away, or go to the nearest hospital emergency room.
What should I avoid while taking aripiprazole orally disintegrating tablets?
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how aripiprazole orally disintegrating tablets affects you. Aripiprazole orally disintegrating tablets may make you drowsy.
- Avoid getting over-heated or dehydrated.
- Do not over-exercise.
- In hot weather, stay inside in a cool place if possible.
- Stay out of the sun. Do not wear too much or heavy clothing.
- Drink plenty of water.
What are the possible side effects of aripiprazole orally disintegrating tablets?
Aripiprazole orally disintegrating tablets may cause serious side effects, including:
- See "What is the most important information I should know about aripiprazole orally disintegrating tablets?"
- Stroke in elderly people (cerebrovascular problems) that can lead to death
- Neuroleptic malignant syndrome (NMS). Tell your healthcare provider right away if you have some or all of the following symptoms: high fever, stiff muscles, confusion, sweating, changes in pulse, heart rate, and blood pressure. These may be symptoms of a rare and serious condition that can lead to death. Call your healthcare provider right away if you have any of these symptoms.
- Uncontrolled body movements (tardive dyskinesia). Aripiprazole orally disintegrating tablets may cause movements that you cannot control in your face, tongue, or other body parts. Tardive dyskinesia may not go away, even if you stop receiving aripiprazole orally disintegrating tablets. Tardive dyskinesia may also start after you stop receiving aripiprazole orally disintegrating tablets.
-
Problems with your metabolism such as:
- High blood sugar (hyperglycemia) and diabetes . Increases in blood sugar can happen in some people who take aripiprazole orally disintegrating tablets. Extremely high blood sugar can lead to coma or death. If you have diabetes or risk factors for diabetes (such as being overweight or a family history of diabetes), your healthcare provider should check your blood sugar before you start aripiprazole orally disintegrating tablets and during your treatment.
▪ feel very thirsty
▪ need to urinate more than usual
▪ feel very hungry
▪ feel weak or tired
▪ feel sick to your stomach
▪ feel confused, or your breath smells fruity
◦ Increased fat levels (cholesterol and triglycerides) in your blood.
◦ Weight gain. You and your healthcare provider should check your weight regularly.
- Unusual urges . Some people taking aripiprazole orally disintegrating tablets have had unusual urges, such as gambling, binge eating or eating that you cannot control (compulsive), compulsive shopping and sexual urges. If you or your family members notice that you are having unusual urges or behaviors, talk to your healthcare provider.
- Orthostatic hypotension (decreased blood pressure). Lightheadedness or fainting may happen when rising too quickly from a sitting or lying position.
- Falls . Aripiprazole orally disintegrating tablets may make you sleepy or dizzy, may cause a decrease in your blood pressure when changing position and can slow your thinking and motor skills which may lead to falls that can cause fractures or other injuries.
- Low white blood cell count
- Seizures (convulsions)
- Problems with control of your body temperature especially when you exercise a lot or are in an area that is very hot. It is important for you to drink water to avoid dehydration. See "What should I avoid while receiving aripiprazole orally disintegrating tablets?"
- Difficulty swallowing that can cause food or liquid to get into your lungs.
The most common side effects of aripiprazole orally disintegrating tablets in adults include:
- Nausea
- vomiting
- constipation
- headache
- blurred vision
- upper respiratory illness
- dizziness
- anxiety
- insomnia
- restlessness
- inner sense of restlessness/need to move (akathisia)
- feeling sleepy
- headache
- vomiting
- fatigue
- increased or decreased appetite
- increased saliva or drooling
- insomnia
- nausea
- stuffy nose
- weight gain
- uncontrolled movement such as restlessness, tremor
- muscle stiffness
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store aripiprazole orally disintegrating tablets?
- Store aripiprazole orally disintegrating tablets at room temperature, between 68°F to 77°F (20°C to 25°C).
Keep aripiprazole orally disintegrating tablets and all medicines out of the reach of children.
General information about the safe and effective use of aripiprazole orally disintegrating tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use aripiprazole orally disintegrating tablets for a condition for which it was not prescribed. Do not give aripiprazole orally disintegrating tablets to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about aripiprazole orally disintegrating tablets that was written for healthcare professionals.
What are the ingredients in aripiprazole orally disintegrating tablets?
Active ingredient: aripiprazole, USP
Inactive ingredients: aspartame, calcium stearate, crospovidone, flavor firmenich powder peppermint and mannitol.
Medication Guide available at www.vionausa.com/medguides or call 1-888-304-5011.
Manufactured by:
Square Pharmaceuticals Ltd.
Dhaka Unit, Kaliakoir, Gazipur-1750, Bangladesh
Distributed by:
Viona Pharmaceuticals Inc.
Cranford, NJ 07016
Rev.: 1/2025
This Medication Guide has been approved by the U.S. Food and Drug Administration.
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELNDC 72578-106-06 - Aripiprazole Orally Disintegrating Tablets, USP 10 mg - 30 Tablets - Rx Only - NDC 72578-107-06 - Aripiprazole Orally Disintegrating Tablets, USP 15 mg - 30 Tablets - Rx ...
Aripiprazole Orally Disintegrating Tablets, USP 10 mg
30 Tablets
Rx Only
Aripiprazole Orally Disintegrating Tablets, USP 15 mg
30 Tablets
Rx Only
Aripiprazole Orally Disintegrating Tablets 10 mg (30 Tablets in 1 Carton)
Each orally disintegrating tablet contains 10 mg aripiprazole USP
NDC 72578-106-78
Rx Only
Aripiprazole Orally Disintegrating Tablets 15 mg (30 Tablets in 1 Carton)
Each orally disintegrating tablet contains 15 mg aripiprazole USP
NDC 72578-107-78
Rx Only
Close
-
INGREDIENTS AND APPEARANCEProduct Information
ARIPIPRAZOLE aripiprazole tablet, orally disintegrating Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72578-106 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARIPIPRAZOLE (UNII: 82VFR53I78) (ARIPIPRAZOLE - UNII:82VFR53I78) ARIPIPRAZOLE 10 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CALCIUM STEARATE (UNII: 776XM7047L) CROSPOVIDONE (UNII: 2S7830E561) PEPPERMINT (UNII: V95R5KMY2B) MANNITOL (UNII: 3OWL53L36A) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) Score no score Shape CAPSULE Size 8mm Flavor PEPPERMINT (FLAVOR FIRMENICH POWDER PEPPERMINT) Imprint Code ZF;41 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72578-106-06 30 in 1 BOTTLE; Type 0: Not a Combination Product 07/20/2022 2 NDC:72578-106-78 3 in 1 CARTON 02/22/2023 2 NDC:72578-106-30 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090165 07/20/2022 ARIPIPRAZOLE aripiprazole tablet, orally disintegrating Product Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72578-107 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARIPIPRAZOLE (UNII: 82VFR53I78) (ARIPIPRAZOLE - UNII:82VFR53I78) ARIPIPRAZOLE 15 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CALCIUM STEARATE (UNII: 776XM7047L) CROSPOVIDONE (UNII: 2S7830E561) PEPPERMINT (UNII: V95R5KMY2B) MANNITOL (UNII: 3OWL53L36A) Product Characteristics Color WHITE (WHITE TO OFF-WHITE) Score no score Shape ROUND Size 8mm Flavor PEPPERMINT (FLAVOR FIRMENICH POWDER PEPPERMINT) Imprint Code ZF;42 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72578-107-06 30 in 1 BOTTLE; Type 0: Not a Combination Product 07/20/2022 2 NDC:72578-107-78 3 in 1 CARTON 02/22/2023 2 NDC:72578-107-30 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090165 07/20/2022 Labeler - Viona Pharmaceuticals Inc. (081468959) Registrant - SQUARE PHARMACEUTICALS PLC. (731487153)
CloseEstablishment Name Address ID/FEI Business Operations SQUARE PHARMACEUTICALS PLC. 850366520 ANALYSIS(72578-106, 72578-107) , LABEL(72578-106, 72578-107) , MANUFACTURE(72578-106, 72578-107) , PACK(72578-106, 72578-107)
Find additional resources
(also available in the left menu)Safety
Boxed Warnings, Report Adverse Events, FDA Safety Recalls, Presence in Breast Milk
Related Resources
Medline Plus, Clinical Trials, PubMed, Biochemical Data Summary
More Info on this Drug
View Labeling Archives, RxNorm, Get Label RSS Feed, View NDC Code(s)NEW!
View Labeling Archives for this drug
ARIPIPRAZOLE tablet, orally disintegrating
Number of versions: 8
RxNorm
ARIPIPRAZOLE tablet, orally disintegrating
Get Label RSS Feed for this Drug
ARIPIPRAZOLE tablet, orally disintegrating
To receive this label RSS feed
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/labelrss.cfm?setid=73c5e380-b7b1-4362-a827-71881574ff48
To receive all DailyMed Updates for the last seven days
Copy the URL below and paste it into your RSS Reader application.
https://dailymed.nlm.nih.gov/dailymed/rss.cfm
What will I get with the DailyMed RSS feed?
DailyMed will deliver notification of updates and additions to Drug Label information currently shown on this site through its RSS feed.
DailyMed will deliver this notification to your desktop, Web browser, or e-mail depending on the RSS Reader you select to use. To view updated drug label links, paste the RSS feed address (URL) shown below into a RSS reader, or use a browser which supports RSS feeds, such as Safari for Mac OS X.
How to discontinue the RSS feed
If you no longer wish to have this DailyMed RSS service, simply delete the copied URL from your RSS Reader.
More about getting RSS News & Updates from DailyMedWhy is DailyMed no longer displaying pill images on the Search Results and Drug Info pages?
Due to inconsistencies between the drug labels on DailyMed and the pill images provided by RxImage, we no longer display the RxImage pill images associated with drug labels.
We anticipate reposting the images once we are able identify and filter out images that do not match the information provided in the drug labels.
NDC Codes
ARIPIPRAZOLE tablet, orally disintegrating
If this SPL contains inactivated NDCs listed by the FDA initiated compliance action, they will be specified as such.
| NDC | |
|---|---|
| 1 | 72578-106-06 |
| 2 | 72578-106-30 |
| 3 | 72578-106-78 |
| 4 | 72578-107-06 |
| 5 | 72578-107-30 |
| 6 | 72578-107-78 |