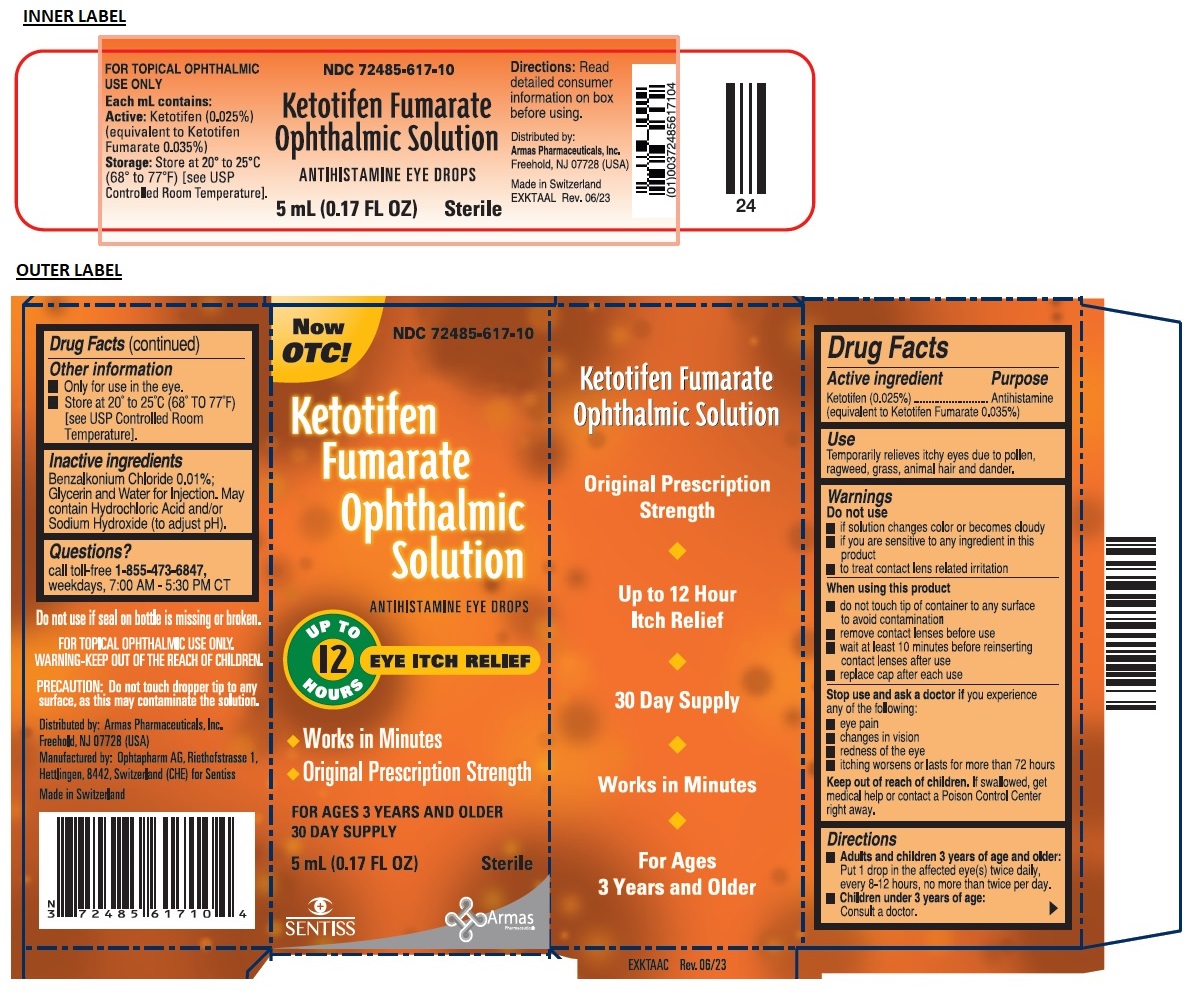
Label: KETOTIFEN FUMARATE OPHTHALMIC SOLUTION- ketotifen fumarate solution/ drops
- NDC Code(s): 72485-617-10
- Packager: Armas Pharmaceuticals Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONDrug Facts
-
Active ingredient
Ketotifen (0.025%) (equivalent to Ketotifen Fumarate 0.035%)
-
Purpose
Antihistamine
-
Use
Temporarily relieves itchy eyes due to pollen, ragweed, grass, animal hair and dander.
-
Warnings
Do not use - if solution changes color or becomes cloudy - if you are sensitive to any ingredient in this product - to treat contact lens related irritation - When using this product - do not touch ...
-
Directions
Adults and children 3 years of age and older: Put 1 drop in the affected eye(s) twice daily, every 8-12 hours, no more than twice per day. Children under 3 years of age: Consult a ...
-
Other information
Only for use in the eye. Store at 20° to 25°C (68° TO 77°F) [see USP Controlled Room Temperature].
-
Inactive ingredients
Benzalkonium Chloride 0.01%; Glycerin and Water for Injection. May contain Hydrochloric Acid and/or Sodium Hydroxide (to adjust pH).
-
Questions?
call toll-free - 1-855-473-6847,weekdays, 7:00 AM - 5:30 PM CT
-
SPL UNCLASSIFIED SECTIONNow OTC! ANTIHISTAMINE EYE DROPS - UPTO 12 HOURS EYE ITCH RELIEF - • Works in Minutes - • Original Prescription Strength - FOR AGES 3 YEARS AND OLDER - 30 DAY SUPPLY - Sterile - Original ...
-
Packaging
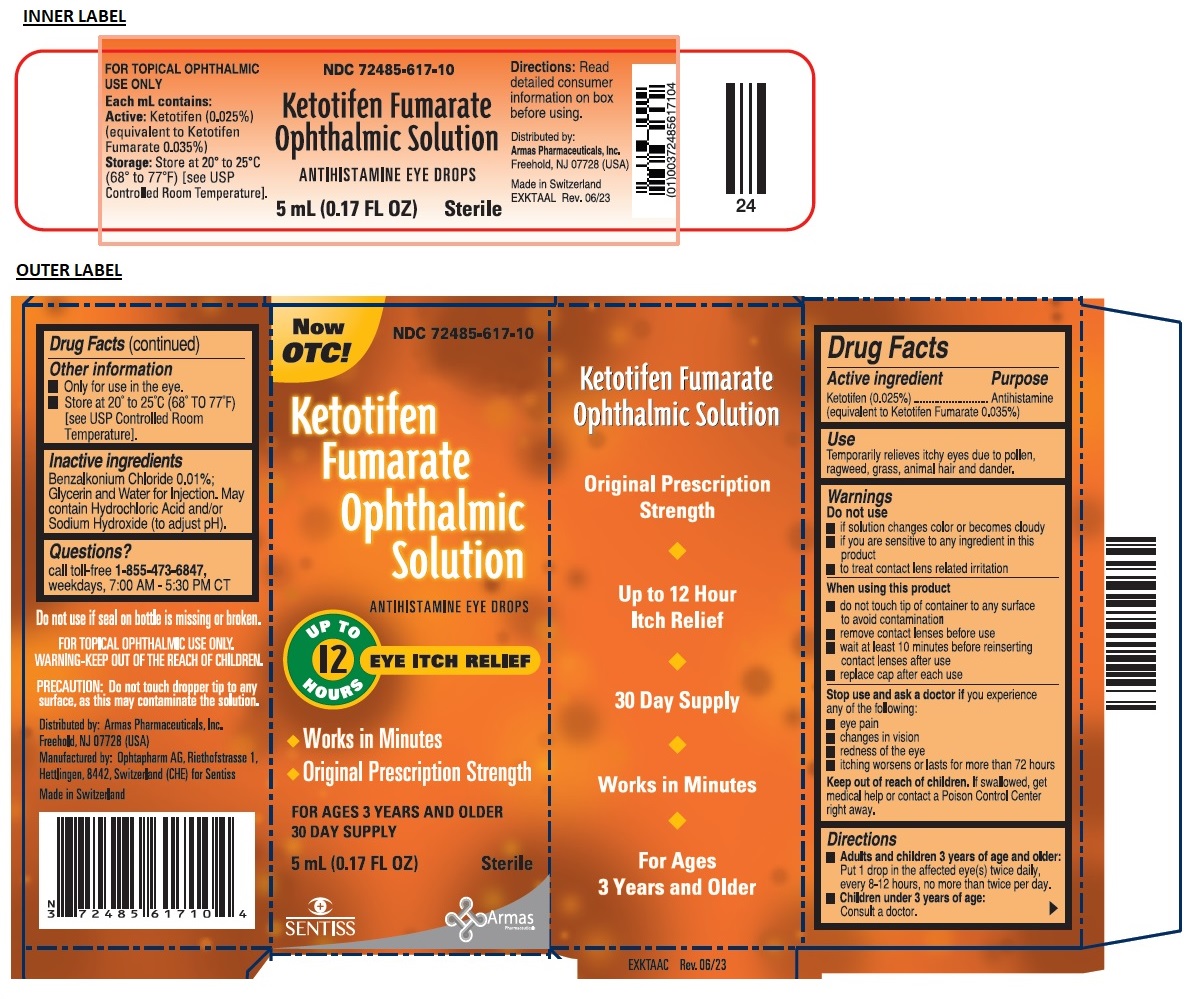
-
INGREDIENTS AND APPEARANCEProduct Information