Label: DORZOLAMIDE HYDROCHLORIDE solution
- NDC Code(s): 72266-197-01
- Packager: FOSUN PHARMA USA INC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated August 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use DORZOLAMIDE HYDROCHLORIDE OPHTHALMIC SOLUTION safely and effectively. See full prescribing information for DORZOLAMIDE HYDROCHLORIDE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGEDorzolamide Hydrochloride Ophthalmic Solution is indicated in the treatment of elevated intraocular pressure in patients with ocular hypertension or open-angle glaucoma.
-
2 DOSAGE AND ADMINISTRATIONThe dose is one drop of Dorzolamide Hydrochloride Ophthalmic Solution in the affected eye(s) three times daily. Dorzolamide Hydrochloride Ophthalmic Solution may be used concomitantly with other ...
-
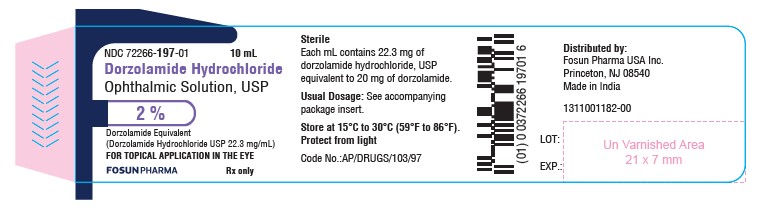
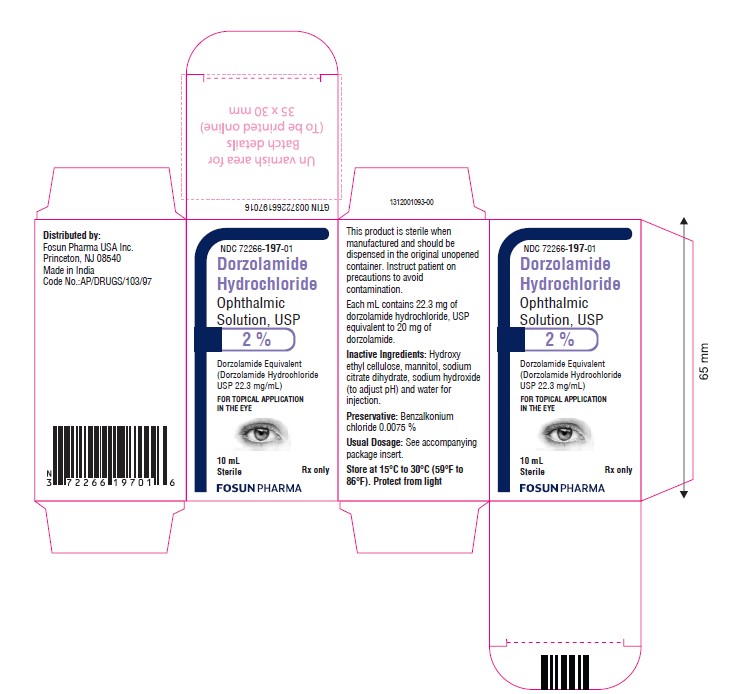
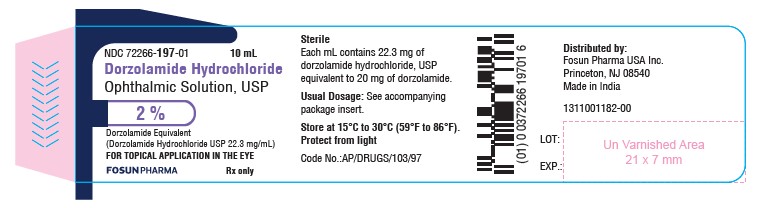
3 DOSAGE FORMS AND STRENGTHSOphthalmic solution containing dorzolamide 2% (20 mg/mL) equivalent to 22.3 mg/mL of dorzolamide hydrochloride, USP.
-
4 CONTRAINDICATIONSDorzolamide Hydrochloride Ophthalmic Solution is contraindicated in patients who are hypersensitive to any component of this product - [see Warnings and Precautions ...
-
5 WARNINGS AND PRECAUTIONS5.1 Sulfonamide Hypersensitivity - Dorzolamide Hydrochloride Ophthalmic Solution contains dorzolamide, a sulfonamide; and although administered topically, it is absorbed systemically. Therefore ...
-
6 ADVERSE REACTIONS6.1 Clinical Trials Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS7.1 Oral Carbonic Anhydrase Inhibitors - There is a potential for an additive effect on the known systemic effects of carbonic anhydrase inhibition in patients receiving an oral carbonic ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Risk Summary - There are no adequate and well-controlled studies in pregnant women with Dorzolamide Hydrochloride Ophthalmic Solution. Dorzolamide caused fetal vertebral ...
-
10 OVERDOSAGEElectrolyte imbalance, development of an acidotic state, and possible central nervous system effects may occur. Serum electrolyte levels (particularly potassium) and blood pH levels should be ...
-
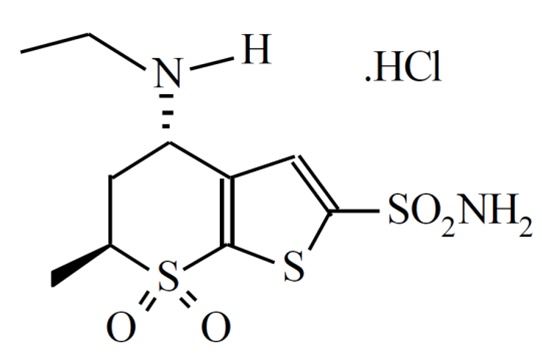
11 DESCRIPTIONDorzolamide Hydrochloride Ophthalmic Solution, USP is a carbonic anhydrase inhibitor formulated for topical ophthalmic use. Dorzolamide hydrochloride, USP is described chemically as ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - Carbonic anhydrase (CA) is an enzyme found in many tissues of the body including the eye. It catalyzes the reversible reaction involving the hydration of carbon dioxide ...
-
13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - In a two-year study of dorzolamide hydrochloride administered orally to male and female Sprague- Dawley rats, urinary bladder ...
-
14 CLINICAL STUDIESThe efficacy of Dorzolamide Hydrochloride Ophthalmic Solution was demonstrated in clinical studies in the treatment of elevated intraocular pressure in patients with glaucoma or ocular ...
-
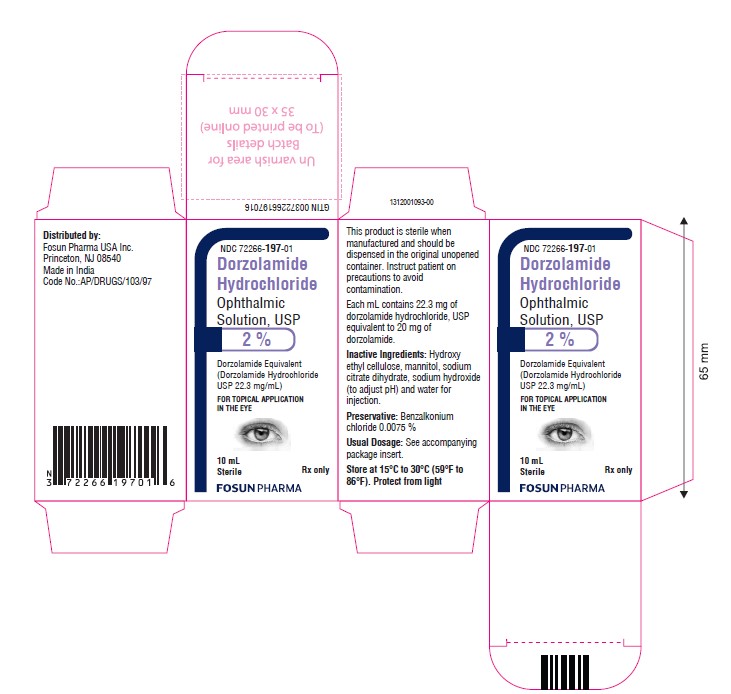
16 HOW SUPPLIED/STORAGE AND HANDLINGDorzolamide Hydrochloride Ophthalmic Solution, USP 2% is supplied in 10 mL ratchet modified screw neck white LDPE bottle with LDPE nozzle having white Opaque pump with a dip tube and Orange color ...
-
17 PATIENT COUNSELING INFORMATIONAdvise the patient to read the FDA-approved patient labeling (Instructions for Use). Sulfonamide Reactions - Dorzolamide Hydrochloride Ophthalmic Solution is a sulfonamide and although ...
-
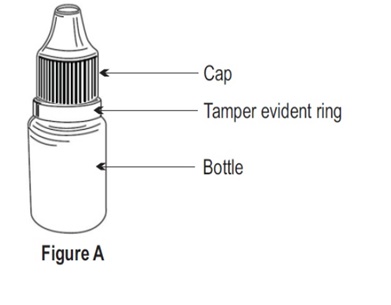
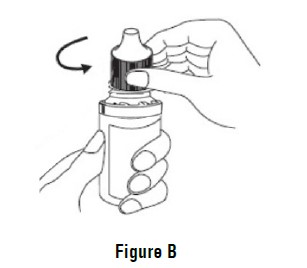
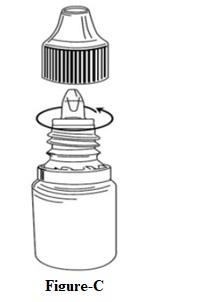
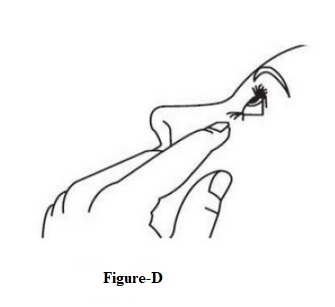
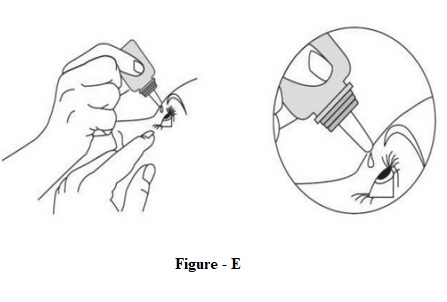
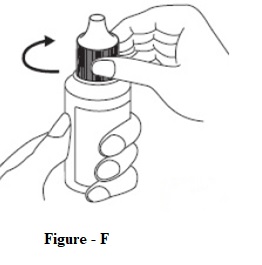
INSTRUCTIONS FOR USEINSTRUCTIONS FOR USE - Dorzolamide Hydrochloride Ophthalmic Solution, USP 2 % (dor ZOL a mide) Before using your dorzolamide hydrochloride ophthalmic solution ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELDorzolamide Hydrochloride Ophthalmic Solution USP, 2.0% NDC 72266-197-01 - Container Label - Carton Label
-
INGREDIENTS AND APPEARANCEProduct Information