Label: HYDROQUINONE cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 72056-060-01 - Packager: Syntenza Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 6, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only - FOR EXTERNAL USE ONLY - NOT FOR OPHTHALMIC USE
-
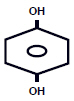
DESCRIPTIONEach gram of Hydroquinone USP, 4% Skin Bleaching Cream contains 40 mg hydroquinone USP, in a vanishing cream base of aqua (water), ascorbic acid, BHT, cetyl alcohol, disodium EDTA, glycerin ...
-
CLINICAL PHARMACOLOGYTopical application of hydroquinone produces a reversible depigmentation of the skin by inhibition of the enzymatic oxidation of tyrosine to 3,4-dihydroxyphenylalanine (dopa) (Denton, C. et al. ...
-
INDICATIONS AND USAGEHydroquinone USP, 4% Skin Bleaching Cream is indicated for the gradual bleaching of hyperpigmented skin conditions such as chloasma, melasma, freckles, senile lentigines, and other unwanted areas ...
-
CONTRAINDICATIONSPrior history of sensitivity or allergic reaction to hydroquinone or to any of the ingredients of the product. The safety of topical hydroquinone use during pregnancy or for children (12 years and ...
-
WARNINGSContains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible ...
-
PRECAUTIONS(see WARNINGS) General - Test for skin sensitivity before using by applying a small amount to an unbroken patch of skin; check within 24 hours. Minor redness is not a contraindication, but where ...
-
ADVERSE REACTIONSThe following adverse reactions have been reported: dryness and fissuring of paranasal and infraorbital areas, erythema, and stinging. Occasional hypersensitivity (localized contact dermatitis ...
-
OVERDOSAGEThere have been no systemic reactions reported from the use of topical hydroquinone. However, treatment should be limited to relatively small areas of the body at one time, since some patients ...
-
DOSAGE AND ADMINISTRATIONHydroquinone USP, 4% Skin Bleaching Cream should be applied to affected areas and rubbed in well twice daily, in the morning and before bedtime, or as directed by a physician. If no improvement is ...
-
HOW SUPPLIEDHydroquinone USP, 4% Skin Bleaching Cream is available as follows: 1 oz (28.35 g) tube (NDC 72056-060-01) STORAGE - Hydroquinone USP, 4% Skin Bleaching Cream should be stored at controlled room ...
-
REFERENCES1 DENTON C., LERNER A.B., FITZPATRICK T.B. Inhibition of Melanin Formation by Chemical Agents - Journal of Investigative Dermatology 1952, 18:119-135. 2 JIMBOW K., OBATA H., PATHAK M., FITZPATRICK ...
-
SPL UNCLASSIFIED SECTIONManufactured for: Syntenza Pharmaceuticals LLC - Edina, MN 55436, USA - Rev. 03/18 - Hydroquinone USP, 4% Skin Bleaching Cream
-
PRINCIPAL DISPLAY PANEL - 28.35 g Tube CartonSYNTENZA - NDC 72056-060-01 - Hydroquinone USP, 4% Skin Bleaching Cream - Net Wt. 1 oz. (28.35 g) Rx Only
-
INGREDIENTS AND APPEARANCEProduct Information