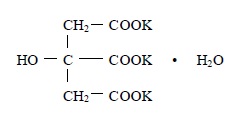Label: POTASSIUM-CIT tablet, extended release
POTASSIUM-CIT tablet, extended release
-
Contains inactivated NDC Code(s)
NDC Code(s): 71930-048-12, 71930-049-12, 71930-050-12 - Packager: Eywa Pharma Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 27, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use POTASSIUM CITRATE EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for POTASSIUM CITRATE ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE1.1 Renal Tubular Acidosis (RTA) with Calcium Stones - Potassium citrate is indicated for the management of renal tubular acidosis [see Clinical Studies (14.1)]. 1.2 Hypocitraturic Calcium ...
-
2 DOSAGE AND ADMINISTRATION2.1 Dosing Instructions - Treatment with extended release potassium citrate should be added to a regimen that limits salt intake (avoidance of foods with high salt content and of added salt at ...
-
3 DOSAGE FORMS AND STRENGTHS5 mEq tablets are uncoated, tan to yellowish speckled in color, round shaped, with E34 debossed on one side and plain on the other side. 10 mEq tablets are uncoated, tan to yellowish speckled in ...
-
4 CONTRAINDICATIONSPotassium citrate extended-release tablets is contraindicated: In patients with hyperkalemia (or who have conditions predisposing them to hyperkalemia), as a further rise in serum potassium ...
-
5 WARNINGS AND PRECAUTIONS5.1 Hyperkalemia - In patients with impaired mechanisms for excreting potassium, potassium citrate administration can produce hyperkalemia and cardiac arrest. Potentially fatal hyperkalemia can ...
-
6 ADVERSE REACTIONS6.1 Postmarketing Experience - Some patients may develop minor gastrointestinal complaints during potassium citrate therapy, such as abdominal discomfort, vomiting, diarrhea, loose bowel ...
-
7 DRUG INTERACTIONS7.1 Potential Effects of Potassium Citrate on Other Drugs - Potassium-sparing Diuretics: Concomitant administration of potassium citrate and a potassium-sparing diuretic (such as triamterene ...
-
8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy - Animal reproduction studies have not been conducted. It is also not known whether potassium citrate can cause fetal harm when administered to a pregnant woman or can affect ...
-
10 OVERDOSAGETreatment of Overdosage: The administration of potassium salts to persons without predisposing conditions for hyperkalemia rarely causes serious hyperkalemia at recommended dosages. It is ...
-
11 DESCRIPTIONPotassium citrate is a citrate salt of potassium. Its empirical formula is K3C6H5O7 • H2O, and it has the following chemical structure: Potassium citrate, USP is a transparent crystals or ...
-
12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action - When potassium citrate is given orally, the metabolism of absorbed citrate produces an alkaline load. The induced alkaline load in turn increases urinary pH and ...
-
14 CLINICAL STUDIESThe pivotal potassium citrate trials were non-randomized and non-placebo controlled where dietary management may have changed coincidentally with pharmacological treatment. Therefore, the results ...
-
16 HOW SUPPLIED/STORAGE AND HANDLINGPotassium Citrate Extended-release Tablets USP, 5 mEq tablets are uncoated, tan to yellowish speckled in color, round shaped, with E34 debossed on one side and plain on the other side, supplied ...
-
17 PATIENT COUNSELING INFORMATION17.1 Administration of Drug - Tell patients to take each dose without crushing, chewing or sucking the tablet. Tell patients to take this medicine only as directed. This is especially ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELPotassium citrate 5 mEq Label: Potassium citrate 10 mEq Label: Potassium citrate 15 mEq Label:
-
INGREDIENTS AND APPEARANCEProduct Information