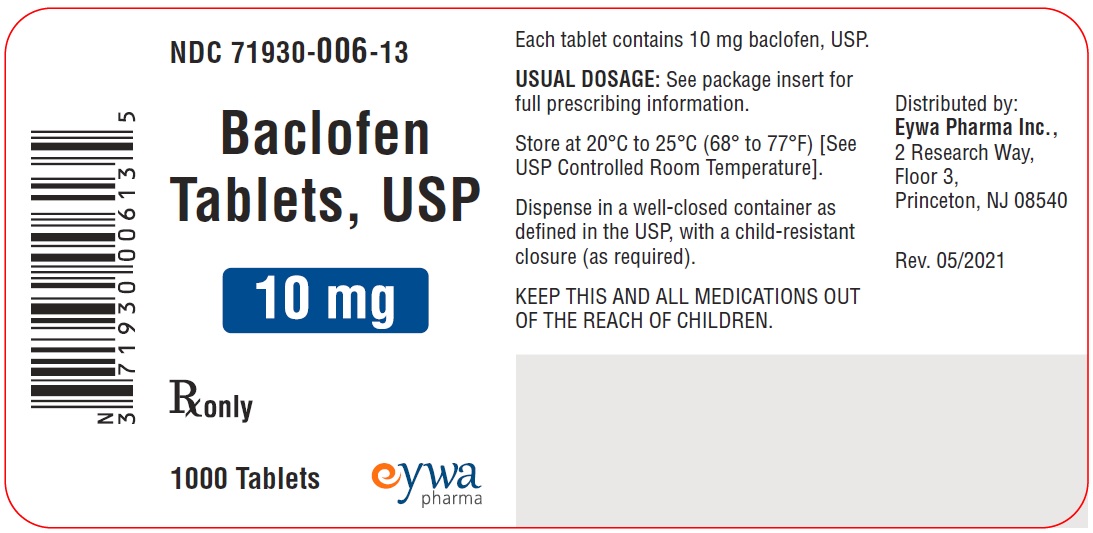
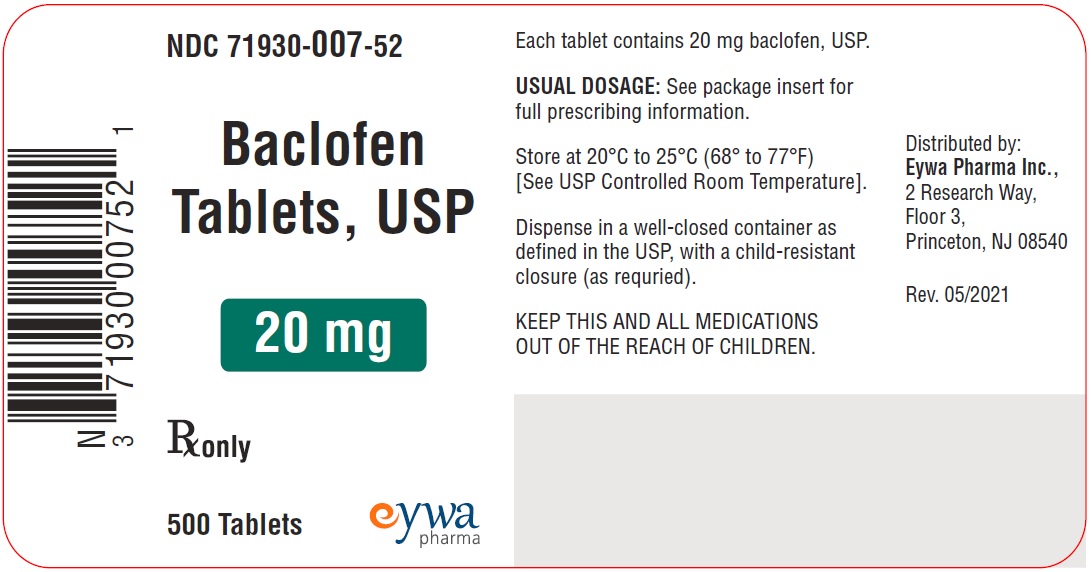
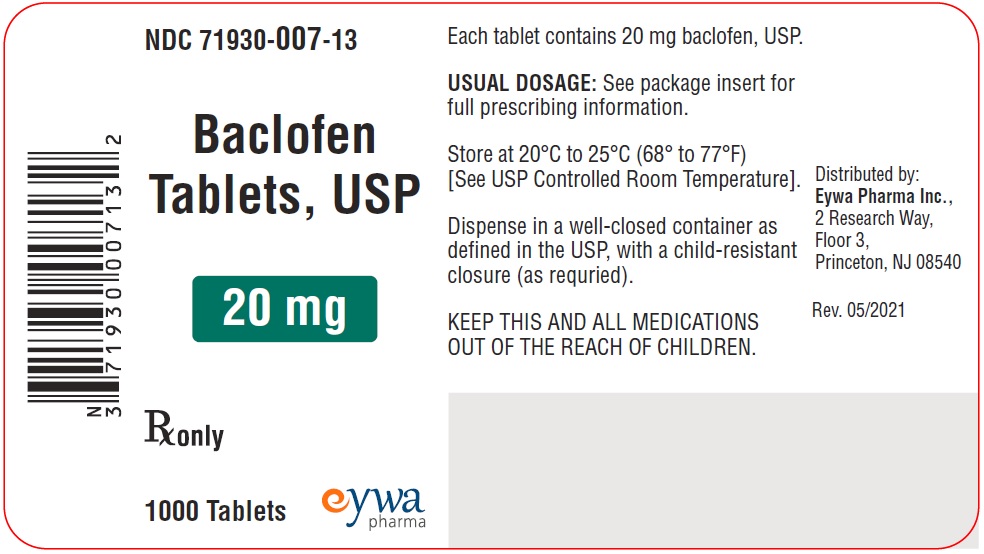
Label: BACLOFEN tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 71930-006-12, 71930-006-13, 71930-006-52, 71930-007-12, view more - Packager: Eywa Pharma Inc
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 11, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
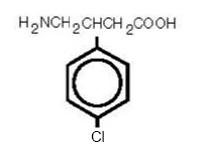
DESCRIPTIONBaclofen USP, is a muscle relaxant and antispastic. Its chemical name is 4-amino-3-(4-chlorophenyl)-butanoic acid. The structural formula is: C10H12ClNO2 M.W. 213.66 - Baclofen USP is a white ...
-
CLINICAL PHARMACOLOGYThe precise mechanism of action of baclofen is not fully known. Baclofen is capable of inhibiting both monosynaptic and polysynaptic reflexes at the spinal level, possibly by hyperpolarization of ...
-
INDICATIONS AND USAGEBaclofen Tablets, USP are useful for the alleviation of signs and symptoms of spasticity resulting from multiple sclerosis, particularly for the relief of flexor spasms and concomitant pain ...
-
CONTRAINDICATIONSHypersensitivity to baclofen.
-
WARNINGSa. Neonatal Withdrawal Symptoms: Withdrawal symptoms have been reported starting hours to days after delivery in neonates whose mothers were treated with oral baclofen throughout pregnancy. The ...
-
PRECAUTIONSBecause of the possibility of sedation, patients should be cautioned regarding the operation of automobiles or other dangerous machinery, and activities made hazardous by decreased alertness ...
-
ADVERSE REACTIONSThe most common is transient drowsiness (10 to 63%). In one controlled study of 175 patients, transient drowsiness was observed in 63% of those receiving baclofen compared to 36% of those in the ...
-
OVERDOSAGESigns and Symptoms: Vomiting, muscular hypotonia, drowsiness, accommodation disorders, coma, respiratory depression, and seizures. Treatment: In the alert patient, empty the stomach promptly ...
-
DOSAGE AND ADMINISTRATIONThe determination of optimal dosage requires individual titration. Start therapy at a low dosage and increase gradually until optimum effect is achieved (usually between 40 to 80 mg daily). The ...
-
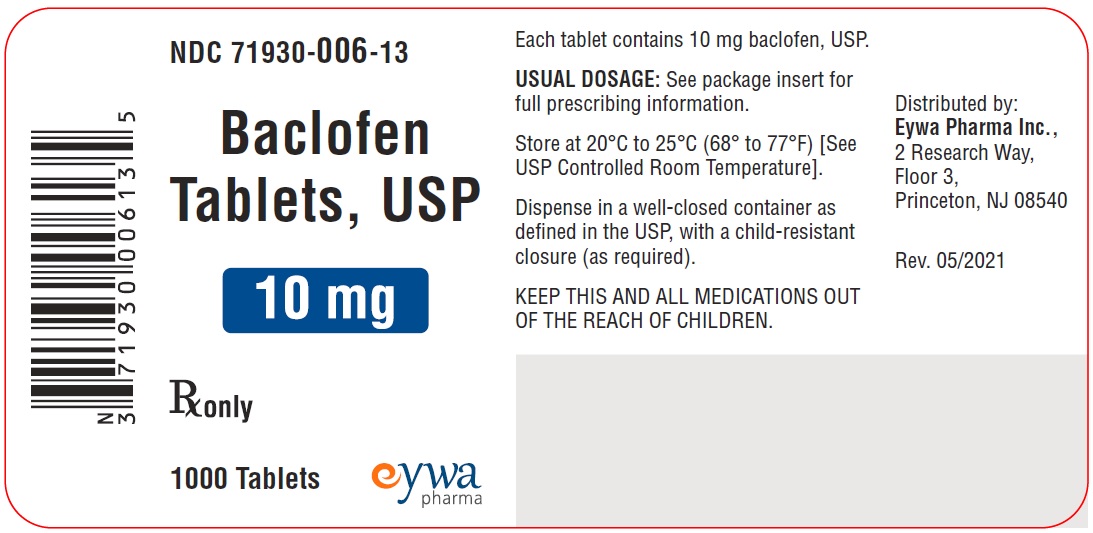
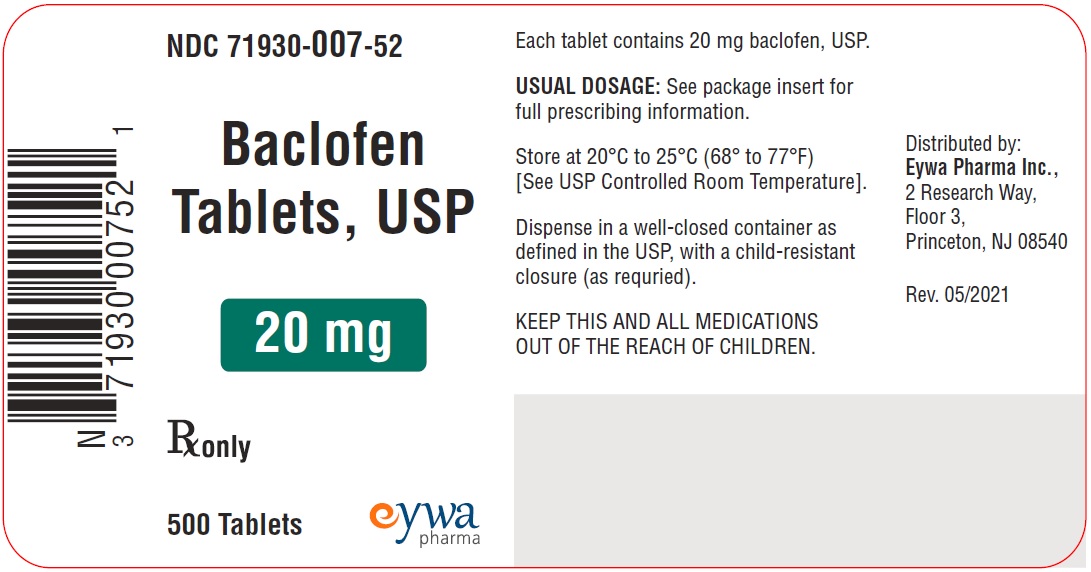
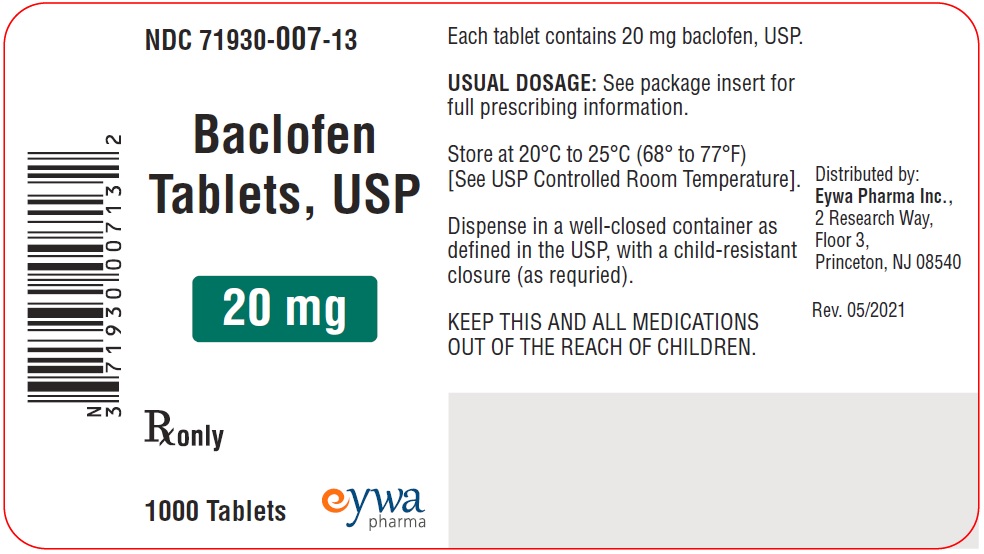
HOW SUPPLIEDFor 5 mg strength: White to off-white, round flat faced beveled edged tablets debossed with ‘E’ on one side and ‘8’ on the other side. They are supplied as follows: NDC 71930-066-12 bottles of ...
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANELBaclofen Tablets USP 5 mg 100s - Baclofen Tablets USP 5 mg 500s - Baclofen Tablets USP 5 mg 1000s - Baclofen Tablets USP 10 mg 100s - Baclofen Tablets USP 10 mg ...
-
INGREDIENTS AND APPEARANCEProduct Information